Linear Methoxy PEGs for PEGylationtest test2022-07-29T09:09:45-06:00
JenKem Technology provides high quality activated methoxy PEGs for PEGylation, with high purity, low polydispersity, and low to no diol content.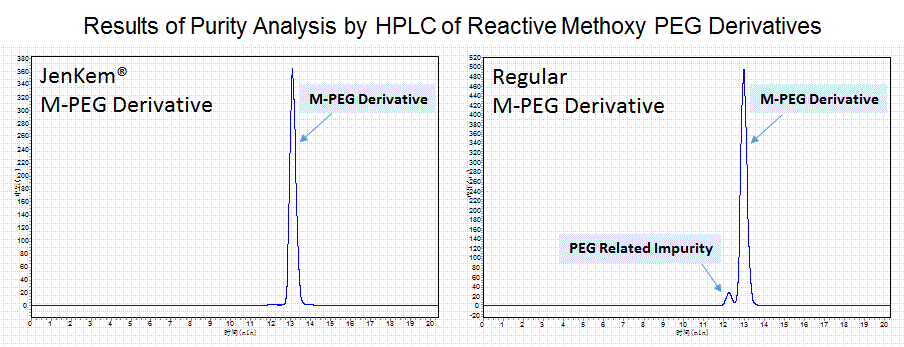
JenKem Technology provides large scale GMP manufacture of high quality linear methoxy PEGs for PEGylation [1] using High Purity (> 99%) Methoxy Polyethylene Glycol (mPEG) Raw Materials. GMP grade PEG derivatives and bulk orders are made-to-order offering the opportunity to match customers’ special quality requirements. GMP grade mPEG derivatives are manufactured from 200g to 40Kg or greater batches, under ISO 9001 and ISO 13485 certified quality management system, following ICH Q7 guidelines.
Methoxy PEGs for PEGylation with molecular weights and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
All other linear monofunctional PEG derivatives have the general structure: CAP―PEG―X, where X is the functional reactive group and CAP can be either a methoxy group, a benzyl group, or a targeting monosaccharide molecule such as glucose, mannose [56], and galactose. For inquiries on cGMP production of PEG derivatives please contact us at tech@jenkemusa.com.
Linear mPEG NHS with Cleavable Linker
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥ 95% |
Methoxy PEG Succinimidyl Succinate is a degradable PEG linker reacting with the amino group of lysine(s) on proteins or other biologics, such as the amines on adenosine deaminase, aspargase, L-asparaginase, at pH 7-8, while the ester linkage is cleaved under regular ester cleaving reaction conditions.[2,3] |
|
≥ 95% |
Methoxy PEG Succinimidyl Glutarate is a degradable PEG linker that reacts with the amino group of lysine(s) on proteins or other biologics at pH 7-8, while the ester linkage is cleaved under regular ester cleaving reaction conditions. |
Linear Monosaccharide PEG NHS
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥ 90% |
Galactose PEG NHS reacts with amino group of lysine(s) at room temperature in under 1hr, at pH 7-8. The presence of the targeting galactose monosaccharide increases significantly the selectivity of the PEGylation reaction. [5] |
|
≥ 90% |
Glucose PEG NHS reacts with amine group of lysine(s) at room temperature in less than 1hr, at pH 7-8. The presence of the targeting sugar group (Glucose) increases significantly the selectivity of the PEGylation reaction. [6] |
Linear mPEG NHS with Stable Linker
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥ 95% |
Methoxy PEG Succinimidyl Carboxymethyl Ester reacts with the amine group of lysine(s) at room temperature in less than 1hr at pH 7-8. Shorter hydrolysis half life of M-PEG-SCM ensures maximum selectivity towards most sterically available amine groups. [7-9, 46, 47] |
|
≥ 95% |
Methoxy PEG Succinimidyl Propionate (Methoxy PEG Succinimidyl Propanoate) reacts with the amine group of lysine(s) [12], such as amines on HGH antagonist, growth hormone B2036, granulocyte colony stimulating factor MAXY-G34 (CSF MAXY-G34), and uricase
|
|
≥ 95% |
Methoxy PEG Succinimidyl Butanoate reacts with the amine group of lysine(s), such as the amines on EPO, at room temperature at pH 7-8. Methoxy PEG Succinimidyl Butanoate has a longer hydrolysis half-life compared with M-PEG-SCM. |
|
≥ 95% |
Methoxy PEG Succinimidyl Hexanoate reacts with the amine group of lysine(s) at pH 7-8. Methoxy PEG Succinimidyl Hexanoate has a longer hydrolysis half-life compared with M-PEG-SCM and M-PEG-SBA. [10, 48, 49] |
|
≥ 95% |
Methoxy PEG Succinimidyl Succinamide reacts with the amine group of lysine(s) at pH 7-8. Methoxy PEG Succinimidyl Succinamide has a longer hydrolysis half-life compared with M-PEG-SCM.[11] |
|
≥ 90% |
Methoxy PEG Succinimidyl Glutaramide reacts with the amine group of lysine(s) at pH 7-8. Methoxy PEG Succinimidyl Glutaramide has a longer hydrolysis half-life compared with M-PEG-SCM. [12] |
Linear mPEG Carbonate
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥ 95% |
Methoxy PEG Succinimidyl Carbonate reacts with the amino group of lysine(s) on proteins or other biologics, such as the amines present on IFN, Interferon alpha 2b. Methoxy PEG Succinimidyl Carbonate has a longer hydrolysis half-life compared with M-PEG-SCM.[4] |
|
≥ 90% |
Methoxy PEG Nitrophenyl Carbonate is reactive towards the the amino group of lysine(s) on proteins or other biologics, with a longer hydrolysis half-life compared with M-PEG-SCM. |
Linear mPEG Carboxyl
Linear Methoxy PEG Amine
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥ 95% |
Methoxy PEG Amine. Attaches via stable linkages, such as amide, urethane, urea, secondary amine; the HCl salt form provides stability for the solid form of M-PEG-NH2 [18, 53, 55] |
Linear Methoxy PEG Aldehyde
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥ 95% |
Methoxy PEG Aldehyde reacts with N-terminal amines, such as the N-terminal on filgastrim gsf, rhGCSF, at pH 5-8 in the presence of a reducing reagent. [19-22]. |
|
≥ 95% |
Methoxy PEG Butyraldehyde reacts with the N-terminal amine on proteins. PEG Butyraldehyde is more stable than PEG Acetaldehyde. |
Linear Methoxy PEG Maleimide
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥ 95% |
Methoxy PEG Maleimide from JenKem Technology is a thiol reactive PEG derivative selective for thiol groups on cystein side chains. Methoxy PEG Maleimide undergoes thiol PEGylation reactions with thiol-containing molecules at pH 5.0-6.5.[23-25] |
Linear Methoxy PEG Vinylsulfone
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥ 90% |
Methoxy PEG Vinylsulfone are high quality products for sulfhydryl PEGylation. Methoxy Vinylsulfone PEG products are special thiol PEGylation PEGs that react at high pH. [26] |
Linear Methoxy PEG Thiol
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥ 95% |
Methoxy PEG Thiol is a high quality activated PEG product for thiol pegylation. Methoxy PEG Thiol PEGylates the thiol groups on cysteine side chains under mild reaction conditions.[27-30] |
Linear Methoxy PEG Iodoacetamide
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥ 90% |
Methoxy PEG Iodoacetamide from JenKem Technology is a PEG derivative reacting with thiol groups on cysteine side chains. |
Biodegradable M-PEG Oligopeptides
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥95% |
Methoxy PEG Di-Glutamic Acid. Oligopeptide PEG for micelle formation used for drug encapsulation and drug delivery |
|
≥90% |
Methoxy PEG Tri-Glutamic Acid. Oligopeptide PEG for micelle formation useful in drug encapsulation and drug delivery |
|
≥90%(Main peak wt%) |
PLL20K-G35-PEG2K, PEG-Poly(L-lysine), Poly(L-lysine), Graft Ratio 3.5, PLL MW 20000, PEG MW 2000 [31-35] |
PEGs for Click Chemistry
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥90% |
Methoxy PEG Alkyne. Click chemistry PEG reagent for reaction with azide groups |
|
≥90% |
Methoxy PEG Azide. The azide group may be reduced to amines by hydrogenolysis. Click PEG reagent for reaction with alkynes, simple reaction conditions, high selectivity, and rapid reaction with high yield. Water can be used as the reaction solvent. [36] |
|
≥90% |
Methoxy PEG DBCO. Methoxy PEG Dibenzocyclooctyne for copper-free click reactions with azides. |
Other Special mPEG Products
| PEG PRODUCT |
SUBSTITUTION |
REACTIVITY DETAILS |
|
≥95% |
Methoxy PEG DMG for lipid nanoparticles (LNPs) and mRNA delivery, such as M-DMG-2000 (Methoxy PEG Dimyristoyl-rac-glycero, MW 2000; DMG-PEG-2000; CAS 1397695-86-1) and related PEG intermediates that are used in COVID-19 vaccines manufacture. |
|
≥99% |
Methoxy PEG DTA , ALC-0159 Methoxy PEG Ditetradecylacetamide for lipid nanoparticles (LNPs) and mRNA drugs and vaccines, such as COVID-19 vaccines. Please contact us for a sample for research purposes. |
|
≥99% |
Methoxy PEG DTDA , Methoxy PEG Ditetradecylamino (M-DTDA-3000, M-DTDA-5000) for lipid nanoparticles (LNPs) and mRNA drugs and vaccines |
|
≥99% |
Methoxy PEG DTDPA , Methoxy PEG Ditetradecylamino (M-DTDPA-2000) for lipid nanoparticles (LNPs) and mRNA drugs and vaccines |
|
≥99% |
Methoxy PEG Glycerol , Methoxy PEG Glycerol (M-GLC-2000) for lipid nanoparticles (LNPs) and mRNA drugs and vaccines, such as COVID-19 vaccines |
|
≥99% |
Methoxy PEG Epoxide , Methoxy PEG Epoxide (M-EPOX-2000) for lipid nanoparticles (LNPs) and mRNA drugs and vaccines, such as COVID-19 vaccines |
|
≥95% |
Methoxy PEG Hydrazide reacts with aldehydes and ketones to give stable hydrazones in a single step; it can also react with activated carboxylic acids [37] |
|
≥90% |
Methoxy PEG Biotin. Avidin reactive PEG. Streptavidin reactive PEG. [38] |
|
≥90% |
Methoxy PEG Silane for glass or silica surface modification and deactivation [39, 40] |
|
≥90% |
Methoxy PEG Acrylate reacts with sulfhydryl groups (Michael addition reaction). Used for Vinyl polymerization or co-polymerization [41] |
|
≥95% |
Methoxy PEG Phosphate. PEG reagent for surface modifications; increases water solubility. [42] |
|
≥90% |
Methoxy PEG Lipoic Acid. (mPEG Thioctic Acid). High affinity for metals, useful for gold nanoparticle surface modification. [43] |
|
≥95% |
Methoxy PEG DSPE mPEG phospholipid for liposome formation. [44, 45] |
|
≥90% |
Methoxy PEG OA mPEG Oleic Acid lipid for liposome formation. |
Linear mPEG Hydroxyl PEG Raw Materials
| PEG RAW MATERIAL |
MAIN PEAK FRACTION BY GPC |
POLYDISPERSITY BY GPC |
|
≥95% |
≤ 1.05-1.10 |
To order directly from JenKem Technology contact us at sales@jenkemusa.com. For global distribution, please visit this link.
References:
- Hutanu, D., et al., Recent Applications of Polyethylene Glycols (PEGs) and PEG Derivatives. Mod Chem appl, 2014, 2(132).
- Mastorakos, P., et al., Biodegradable DNA Nanoparticles that Provide Widespread Gene Delivery in the Brain. Small, 2015.
- Jain, S., et al., Combinatorial bio-conjugation of gemcitabine and curcumin enables dual drug delivery with synergistic anticancer efficacy and reduced toxicity, RSC Adv., 2014, 4, 29193-29201.
- Vaillard, V.A., et al., mPEG–NHS carbonates: Effect of alkyl spacers on the reactivity: Kinetic and mechanistic insights, Journal of Applied Polymer Science, 2019, 136(5):47028.
- Luan, J., et al., GSDMD membrane pore is critical for IL-1β release and antagonizing IL-1β by hepatocyte-specific nanobiologics is a promising therapeutics for murine alcoholic steatohepatitis, Biomaterials, 2020, V. 227.
- Wei, G, et al., Glucose Transporter 1 (GLUT1)-Targeting and Hypoxia-Activated Mitochondria-Specific Chemo-thermal Therapy via a Glycosylated Poly (amido amine)/Celastrol (PAMAM/Cel) Complex. Journal of Colloid and Interface Science. 2021.
- Sousa, A., et al., Design of experiments to select triphenylphosphonium-polyplexes with suitable physicochemical properties for mitochondrial gene therapy, Journal of Molecular Liquids, 2020, V. 302.
- Yan, J., et al., Tumor Contrast Imaging with Gas Vesicles by Circumventing the Reticuloendothelial System, Ultrasound in Medicine & Biology, 2020, V. 46(2); P. 359-368.
- Chen, L., Ultra-small MoS2 nanodots-incorporated mesoporous silica nanospheres for pH-sensitive drug delivery and CT imaging, Journal of Materials Science & Technology, 2021, V. 63, P. 91-96.
- Mesken, J., et al., Modifying plasmid-loaded HSA-nanoparticles with cell penetrating peptides–Cellular uptake and enhanced gene delivery, International journal of pharmaceutics, 2017, 522(1):198-209.
- Li, C., et al., Real-Time Monitoring Surface Chemistry-Dependent In Vivo Behaviors of Protein Nanocages via Encapsulating an NIR-II Ag2S Quantum Dot, ACS Nano, 2015, 9 (12), 12255-12263.
- Supasena, W., et al., Enhanced selective cytotoxicity of doxorubicin to breast cancer cells by methoxypolyethylene glycol conjugation via a novel beta-thiopropanamide linker, European Polymer Journal, 2020, 141, 110056.
- Pfister, D., et al., Integrated process for high conversion and high yield protein PEGylation, Biotechnol. Bioeng., 2016.
- Goffin V, et al., Pegvisomant Pfizer/Sensus. Curr Opin Investig Drugs, 2004, 5:463–468.
- https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI%20Thesaurus&code=C71718.
- Lassenberger, A., et al., Individually stabilized, superparamagnetic nanoparticles with controlled shell and size leading to exceptional stealth properties and high relaxivities. ACS Applied Materials & Interfaces, 2017.
- Liu, Y., et al., Cathodic protected Mn2+ by NaxWO3 nanorods for stable magnetic resonance imaging-guided tumor photothermal therapy, Biomaterials, 2020, V. 234.
- Xia, C., et al., Redox-responsive nanoassembly restrained myeloid-derived suppressor cells recruitment through autophagy-involved lactate dehydrogenase A silencing for enhanced cancer immunochemotherapy, Journal of Controlled Release, 2021, V. 335, P. 557-574.
- Kateja, N., et al., Development of an integrated continuous PEGylation and purification Process for granulocyte colony stimulating factor, Journal of Biotechnology, 2020, 322, p. 79-89.
- Hebbi, V., et al., Process analytical technology application for protein PEGylation using near infrared spectroscopy: G-CSF as a case study, Journal of Biotechnology, 2021, 325, P. 303-311.
- Luo, S., et al., A new site-specific monoPEGylated β-lactoglobulin at the N-terminal: Effect of different molecular weights of mPEG on its conformation and antigenicity, Food Chemistry, 2021, 343, 128402.
- http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000420/WC500025941.pdf.
- Umeshappa, C. S., et al., Liver-specific T regulatory type-1 cells program local neutrophils to suppress hepatic autoimmunity via CRAMP, Cell Reports, 2021, V. 34(13).
- Kaminskas, L.M., et al., A 30 kDa polyethylene glycol-enfuvirtide complex enhances the exposure of enfuvirtide in lymphatic viral reservoirs in rats, European Journal of Pharmaceutics and Biopharmaceutics, 2019.
- Shen, L., et al., Facile synthesis of organosilica-capped mesoporous silica nanocarriers with selective redox-triggered drug release properties for safe tumor chemotherapy, Biomaterials science, 2019.
- Mahou, R., et al., Injectable and inherently vascularizing semi-interpenetrating polymer network for delivering cells to the subcutaneous space, Biomaterials, 2017.
- Guo, T., et al., Highly-selective detection of EGFR mutation gene in lung cancer based on surface enhanced Raman spectroscopy and asymmetric PCR, Journal of Pharmaceutical and Biomedical Analysis, 2020, 190, 113522.
- Wang, X.-M., et al., Exposure-time-dependent subcellular staging of gold nanoparticles deposition and vesicle destruction in mice livers, Nanomedicine: Nanotechnology, Biology and Medicine, 2021, V. 34.
- Adibnia, V., et al., Chitosan hydrogel micro-bio-devices with complex capillary patterns via reactive-diffusive self-assembly, Acta Biomaterialia, 2019.
- Zhang, Y., et al., DNA-assembled visible nanodandelions with explosive hydrogen-bond breakage achieving uniform intra-tumor distribution (UITD)-guided photothermal therapy, Biomaterials, 2022, 121381.
- Schaedel, L., et al., Microtubules self-repair in response to mechanical stress, Nature Mater., 2015, 14(11):1156-63.
- Bhajun, R., et al., A statistically inferred microRNA network identifies breast cancer target miR-940 as an actin cytoskeleton regulator, Scientific Reports, 2015, 5: 8336.
- Portran, D., Micropatterning Microtubules, Methods in Cell Biology, 2014, 39-51.
- Boujemaa-Paterski, R., et al., Directed actin assembly and motility, Methods Enzymol, 2014, 540: 283-300.
- Vignaud, T., et al., Polyacrylamide hydrogel micropatterning. Methods Cell Biol, 2014, 120: 93-116.
- Li, J., et al., Smart Asymmetric Vesicles with Triggered Availability of Inner Cell-Penetrating Shells for Specific Intracellular Drug Delivery, ACS Applied Materials & Interfaces, 2017.
- Zhou, Z., et al., Comparison of Site-Specific PEGylations of the N-Terminus of Interferon Beta-1b: Selectivity, Efficiency, and in Vivo/Vitro Activity. Bioconjugate Chemistry, 2013, 25(1): p. 138-146.
- Wu, Y., et al., Fabrication of thermo-sensitive complex micelles for reversible cell targeting, Journal of Materials Science: Materials in Medicine, 2015, 26:255.
- Nakashima, K. K., et al., Chapter Thirteen – Enzymatic control over coacervation, Methods in Enzymology, Academic Press, 2021, 646, P. 353-389.
- Yu, L., et al., Ultrasmall mesoporous organosilica nanoparticles: Morphology modulations and redox-responsive biodegradability for tumor-specific drug delivery, Biomaterials, 2018, V. 161, P. 292-305.
- Carleton, M. M., et al., Injectable and degradable methacrylic acid hydrogel alters macrophage response in skeletal muscle, Biomaterials, 2019, 223.
- Gonzalez-Villegas, J., et al., Poly (ethylene glycol)-modified zirconium phosphate nanoplatelets for improved doxorubicin delivery, Inorganica Chimica Acta, 2017.
- Limor Minai, et al., Optical Nanomanipulations of Malignant Cells: Controlled Cell Damage and Fusion, Small, 2012.
- Liu, M., et al., Evasion of the accelerated blood clearance phenomenon by branched PEG lipid derivative coating of nanoemulsions, International Journal of Pharmaceutics, 2022, 612:121365.
- Liu, M., et al., Branched PEG-modification: A new strategy for nanocarriers to evade of the accelerated blood clearance phenomenon and enhance anti-tumor efficacy, Biomaterials, 2022, 283, p.121415.
- Wang, P., et al., Precise gene delivery systems with detachable albumin shell remodeling dysfunctional microglia by TREM2 for treatment of Alzheimer’s disease, Biomaterials, 2022, V. 281.
- Moussa, A., et al., Reducing-end “clickable” functionalizations of chitosan oligomers for the synthesis of chitosan-based diblock copolymers, Carbohydrate Polymers, 2019, V. 219, P. 387-394.
- Look, L., et al., Ligand-Modified Human Serum Albumin Nanoparticles for Enhanced Gene Delivery, Molecular Pharmaceutics, 2015, 12 (9), 3202-3213.
- Gossmann, R., et al., Comparative examination of adsorption of serum proteins on HSA- and PLGA-based nanoparticles using SDS–PAGE and LC–MS, European Journal of Pharmaceutics and Biopharmaceutics, 2015, V. 93, p. 80-87.
- Zhao, L., et al., An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. Journal of Controlled Release, 2017.
- Javanmardi, S., et al., Redox-Sensitive, PEG-Shielded Carboxymethyl PEI Nanogels Silencing MicroRNA-21, Sensitizes Resistant Ovarian Cancer Cells to Cisplatin, Asian Journal of Pharmaceutical Sciences, 2018.
- Brinkman, A.M., et al., Aminoflavone-loaded EGFR-targeted unimolecular micelle nanoparticles exhibit anti-cancer effects in triple negative breast cancer. Biomaterials, 2016, 101:20-31.
- Lv, F., et al., Enhanced mucosal penetration and efficient inhibition efficacy against cervical cancer of PEGylated docetaxel nanocrystals by TAT modification, Journal of Controlled Release, 2021, V. 336, P. 572-582.
- Oddone, N., et al., ROS-responsive “smart” polymeric conjugate: Synthesis, characterization and proof-of-concept study, International Journal of Pharmaceutics, 2019, 570.
- Lin, M, et al., Photo-Triggered Polymeric Antimicrobial Peptide Mimics with Excellent Selectivity and Synchronizing Antifouling and Antimicrobial Hydrogels, Giant, 2022.
- He, H., et al., Nanoparticle-based “Two-pronged” approach to regress atherosclerosis by simultaneous modulation of cholesterol influx and efflux, Biomaterials, 2020, 260.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7 guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.

