JenKem Technology provides PEGylation services up to pre-clinical stage, and custom synthesis of PEGylated conjugates. PEGylation is a versatile tool to improve the water solubility, biocompatibility, immunogenicity, and the PK/PD profile of drugs. JenKem Technology’s dedicated and experienced PEGylation group offers two service models to meet your unique PEGylation needs for proteins, peptides, oligonucleotides, and small molecules.
JenKem Technology has patented many PEG-related technologies and respects the intellectual property of others. As of June 2024, JenKem Technology has 109 active patents, 87 pending patent applications, and many other innovative technologies under development. Several of these technologies are offered for out-licensing: https://www.jenkemusa.com/out-licensing-opportunities.
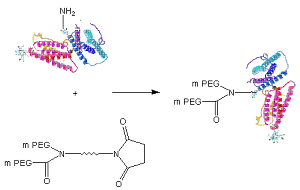
Schematic of protein PEGylation with JenKem® patented Y-Shape PEG NHS
PEGylation Service Model 1
Custom synthesis of PEGylated conjugates, PEG-proteins, PEG-peptides, PEG-polypeptides, PEG-oligonucleotides, PEG-small molecules, and more, using JenKem® catalog PEGs or custom PEG products. JenKem Technology will PEGylate your own protein, peptide, oligonucleotide, or small molecule, and deliver your PEGylated product with a certificate of analysis as a regular end-product, for further testing at your site. This is recommended for an initial evaluation of PEGylation approaches.
PEGylation Service Model 2
Complete PEGylation method development service. Under this service model, JenKem Technology will provide your PEGylated sample, as well as the complete method development package, including developed analytical methods for characterization of your PEGylated product, up to pre-clinical stage. The dedicated PEGylation group can help you identify the right high quality PEG derivatives for your project. Processes developed by JenKem Technology are easily scalable and transferable.
Phase 1: JenKem Technology delivers PEGylated compounds to the customer for pharmaceutical analysis and provides a detailed report including PEGylation reaction conditions, separation and purification processes, and analytical methods for the PEGylated compounds.
- PEG reagent screening
- Screening of PEGylation reaction conditions
- PEGylation process development, including purification
- Analytical method development
- Synthesis, characterization and delivery of the PEGylated molecule
Phase 2: JenKem Technology coordinates the IND enabling preclinical study data for PEGylated compounds together with partner CROs, to determine the optimal PEGylated compounds for customers’ application.
- Quality specification study of PEGylated molecules
- Formulation study of PEGylated molecules
- Pharmacokinetics and pharmacodynamics (PK/PD) study of PEGylated molecules
- Toxicology study of PEGylated molecules
- IND filing with China FDA
For detailed information about our custom PEGylation services, please contact us at tech@jenkemusa.com.
Example PEGylation Projects
IND Approval Received for PEG-irinotecan Developed by JenKem Technology Co., Ltd. – China FDA approved 3SBio Inc.’s Investigational New Drug (IND) appplication for PEG-irinotecan. 3SBio Inc., a leading biotechnology company in China entered into an exclusive license agreement with JenKem Technology Co., Ltd. for the development, manufacturing and marketing in Mainland China of PEG-irinotecan. 3SBio Inc. intends to develop PEG-irinotecan for metastatic breast cancer and colorectal cancer, and platinum-resistant ovarian cancer and will undergo clinical trials in China. Read More
Out-Licensing Opportunities
We are pleased to offer several other innovative PEG and PEGylation-related technologies for out-licensing as sole and non-exclusive licenses. Please click on the link below for details.
Link to Out-Licensing Opportunities – JenKem Technology USA (jenkemusa.com)
References:
- Song S, Sun D, Wang H, Wang J, Yan H, Zhao X, Fawcett JP, Xu X, Cai D, Gu J. Full-profile pharmacokinetics, anticancer activity and toxicity of an extended release trivalent PEGylated irinotecan prodrug. Acta Pharmaceutica Sinica B. 2023 Jan 10.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7 guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.
