PEG products with additional MW may be made to order, please contact us for details
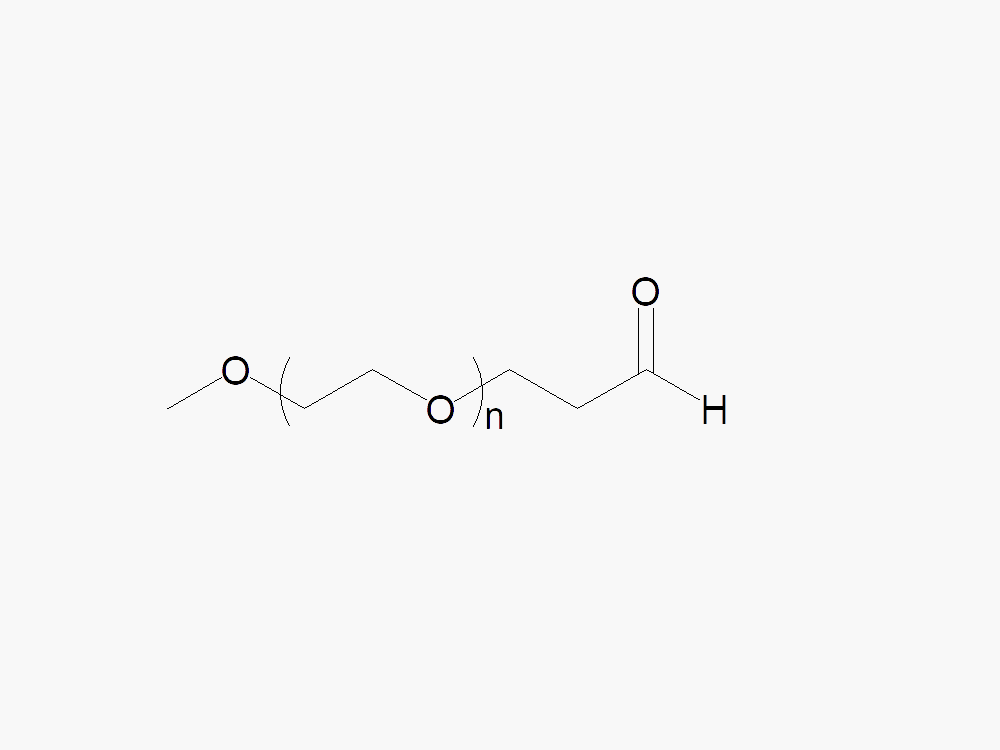
Methoxy PEG Propionaldehyde
$120.00 – $1,200.00
Description
Methoxy PEG Propionaldehyde with superior quality specification of ≥95% Substitution.
Methoxy PEG Propionaldehyde is an N-terminal amine reactive PEG. Methoxy PEG Propionaldehyde reacts with N-terminal amines, such as the N-terminal on gsf, rhGCSF, at pH 5-8 in the presence of a reducing reagent. [45].
JenKem Technology offers Methoxy PEG Propionaldehyde with MW 5kDa (M-ALD-5000), 10kDa (M-ALD-10K), 20kDa (M-ALD-20K), 30kDa (M-ALD-30K) and 40kDa (M-ALD-40K), in 1g and 10g packing sizes. Different MW of Methoxy Propionaldehyde PEG products may be available by custom synthesis, please email us at tech@jenkemusa.com for details on custom PEGs. JenKem Technology provides repackaging services for an additional fee, please contact us if you require a different package size than our catalog selection.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS for Methoxy PEG Propionaldehyde
References:
- Hebbi, V., et al., Process analytical technology application for protein PEGylation using near infrared spectroscopy: G-CSF as a case study, Journal of Biotechnology, 2021, 325, P. 303-311.
- Luo, S., et al., A new site-specific monoPEGylated β-lactoglobulin at the N-terminal: Effect of different molecular weights of mPEG on its conformation and antigenicity, Food Chemistry, 2021, 343, 128402.
- Kateja, N., et al., Development of an integrated continuous PEGylation and purification Process for granulocyte colony stimulating factor, Journal of Biotechnology, 2020, 322, p. 79-89.
- Zaykov, A. N., et al., Insulin-like peptide 5 fails to improve metabolism or body weight in obese mice, Peptides, 2019, 120.
- Zaghmi, A., et al., Determination of the degree of PEGylation of protein bioconjugates using data from proton nuclear magnetic resonance spectroscopy, Data in Brief, V. 25, 2019, 104037.
- Zaghmi, A., et al., Mechanisms of activity loss for a multi-PEGylated protein by experiment and simulation, Materials Today Chemistry, 2019, 12:121-31.
- Hernandez-Vargas, G., et al., Thermo-separating polymer-based aqueous two-phase systems for the recovery of PEGylated lysozyme species, Journal of Chromatography B., 2019, 1105:120-8.
- Cheng, Y., et al., Doxorubicin Loaded Tumor-Triggered Targeting Ammonium Bicarbonate Liposomes for Tumor-Specific Drug Delivery, Colloids and Surfaces B: Biointerfaces, 2019.
- Gertler, A., et al., Pegylated Human Leptin D23L Mutant–Preparation and Biological Activity In Vitro and in Vivo in Male ob/ob Mice, Endocrinology, 2019.
- Liu, S., et al., Acetazolamide‐Loaded pH‐Responsive Nanoparticles Alleviating Tumor Acidosis to Enhance Chemotherapy Effects, Macromolecular bioscience, 2019, 9(2).
- Abbasi, S., et al., Polyacrylamide–b-copolypeptide hybrid copolymer as pH-responsive carrier for delivery of paclitaxel: Effects of copolymer composition on nanomicelles properties, loading efficiency and hemocompatibility, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, v. 537, p. 217-226.
- Behi, J., et al., Optimization of PEGylation reaction time and molar ratio of rhG-CSF toward increasing bioactive potency of monoPEGylated protein, International Journal of Biological Macromolecules, 2018, V. 109, P. 888-895.
- Mejia‐Manzano, L.A., et al., Optimized purification of mono‐PEGylated lysozyme by Heparin Affinity Chromatography using Response Surface Methodology, Journal of Chemical Technology and Biotechnology, 2017.
- Zhao, Y.Z., et al., PEGylation with the thiosuccinimido butylamine linker significantly increases the stability of haloalkane dehalogenase DhaA, Journal of Biotechnology, 2017.
- Mejia‐Manzano, L.A., et al., Recovery of PEGylated and native lysozyme using an in situ aqueous two‐phase system directly from the PEGylation reaction, Journal of Chemical Technology and Biotechnology, 2017.
- Zhang, L., et al., Suppression for lung metastasis by depletion of collagen I and lysyl oxidase via losartan assisted with paclitaxel-loaded pH-sensitive liposomes in breast cancer, Drug Delivery, 2016.
- Mayolo‐Deloisa, K., et al., PEGylated protein separation using different hydrophobic interaction supports: Conventional and monolithic supports. Biotechnology progress, 2016.
- Zhang, Y., et al., Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Scientific Reports, 2016, 6:21225.
- Mata-Gomez, M.A., et al., Modelling of electrokinetic phenomena for capture of PEGylated ribonuclease A in a microdevice with insulating structures, Biomicrofluidics, 2016, 10(3): 033106.
- , et al., Design and cell cytotoxicity assessment of palmitoylated polyethylene glycol-grafted chitosan as nanomicelle carrier for paclitaxel. J. Appl. Polym. Sci., 2015, 133, 43233.
- Mayolo-Deloisa, K., et al., Aldehyde PEGylation of laccase from Trametes versicolor in route to increase its stability: effect on enzymatic activity, Journal of Molecular Recognition, 2015, 28(3): 173-179.
- Wu, L., et al., N-Terminal Modification with Pseudo-Bifunctional PEG-Hexadecane Markedly Improves the Pharmacological Profile of Human Growth Hormone, Molecular Pharmaceutics, 2015.
- Zhang, L., et al., High Tumor Penetration of Paclitaxel Loaded pH Sensitive Cleavable Liposomes by Depletion of Tumor Collagen I in Breast Cancer, ACS Applied Materials & Interfaces, 2015.
- Mata-Gómez, M. A., et al., Dielectrophoretic behavior of PEGylated RNase A inside a microchannel with diamond-shaped insulating posts. Electrophoresis, 2015.
- Parikh, H., et al., Improving Properties of Recombinant SsoPox by Site-Specific Pegylation, Protein and Peptide Letters, 2015, 22:12.
- Tiwari, D., et al., Efficient Purification of rhG-CSF and its PEGylated Forms and Evaluation for In Vitro Activities, Protein and Peptide Letters, 2015, 22:10, pp. 877-884(8).
- Schulz, J. D., Site-Specific Polymer Conjugation Stabilizes Therapeutic Enzymes in the Gastrointestinal Tract. Adv. Mater., 2015.
- Wu, L., Phenyl Amide Linker Improves the Pharmacokinetics and Pharmacodynamics of N-Terminally Mono-PEGylated Human Growth Hormone, Mol. Pharmaceutics, 2014, 11(9), p: 3080–3089.
- Xue, X., et al., Heat treatment increases the bioactivity of C-terminally PEGylated staphylokinase. Process Biochemistry, 2014. 49(7): p. 1092-1096.
- Pink, A., et al., Purification, characterization and plasma half-life of PEGylated soluble recombinant non-HA-binding CD44, BioDrugs, 2014, 28(4) p:393-402.
- Yu, W., et al., PEGylated recombinant human interferon-ω as a long-acting antiviral agent: Structure, antiviral activity and pharmacokinetics. Antiviral Research, 2014, 108: p. 142-147.
- Li, R., et al., Preparation and Characterization of Biological Non-toxic Hybrid Nanoparticles Based on Lactide and Poly(ethylene glycol) Loading Docetaxel for Anticancer Drug Delivery, Chinese Journal of Chemical Engineering, 2014, 22:11–12, P. 1357-1362.
- Martinez, A.H., et al., Chemical grafting of Sepharose 6B and its use in the purification of PEGylated RNase A, Separation and Purification Technology, 2014, 136, P. 190-198.
- Mu Q, et al., Molecular Insight into the Steric Shielding Effect of PEG on the Conjugated Staphylokinase: Biochemical Characterization and Molecular Dynamics Simulation, PLoS ONE, 2013, 8(7): e68559.
- Wu, L., et al., N-terminal mono-PEGylation of growth hormone antagonist: Correlation of PEG size and pharmacodynamic behavior, International journal of pharmaceutics, 2013, 453.2 : 533-540.
- Tian, H., et al., PEGylation enhancement of pH stability of uricase via inhibitive tetramer dissociation, Journal of Pharmacy and Pharmacology, 2013, 65.1 : 53-63.
- Galindo‐López, M., et al., Practical non‐chromatography strategies for the potential separation of PEGylated RNase A conjugates, Journal of Chemical Technology and Biotechnology, 2013, 88.1 : 49-54.
- Gonzalez‐Valdez, J., et al., Effects of chemical modifications in the partition behavior of proteins in aqueous two‐phase systems: A case study with RNase A., Biotechnology progress, 2013, 29.2 : 378-385.
- Niv-Spector, L., Preparation and characterization of mouse IL-22 and its four single-amino-acid muteins that act as IL-22 receptor-1 antagonists, Protein Engineering, Design & Selection, 2012, 25(8). p:397-404.
- Mayolo-Deloisa, C., et al., Hydrophobic interaction chromatography for purification of monoPEGylated RNase A, J. Chromatogr. A, 2012, 1242 p: 11–16.
- Niv-Spector, L., et al., Large-scale preparation and characterization of non-pegylated and pegylated superactive ovine leptin antagonist, Protein Expression and Purification, 2012, 81:2, p. 186-192.
- Gonzalez-Valdez , J., et al., Quantification of RNase A and Its PEGylated Conjugates on Polymer-Salt Rich Environments Using UV Spectrophotometry, Analytical Letters, 2011, 44:5.
- Shpilman, M., et al., Development and Characterization of High Affinity Leptins and Leptin Antagonists, The Journal of Biological Chemistry, 2011, 286(6), p: 4429 –4442.
- Top, A., et al., Conformational and Aggregation Properties of a PEGylated Alanine-Rich Polypeptide, Biomacromolecules, 2011, 12(6), pp 2184–2192.
- Wang, J., et al., Kinetic and stoichiometric analysis of the modification process for N-terminal PEGylation of staphylokinase, Analytical Biochemistry, 2011, 412: 1, P. 114-116.
- Gonzalez-Valdez, J., et al., Potential application of aqueous two-phase systems for the fractionation of RNase A and α-Lactalbumin from their PEGylated conjugates, J. Chem. Technol. Biotechnol., 2011, 86: 26–33.
- Wang, Y-J., et al., PEGylation markedly enhances the in vivo potency of recombinant human non-glycosylated erythropoietin: A comparison with glycosylated erythropoietin, Journal of Controlled Release, 2010, 145:3, p. 306-313.
- Elinav, E., et al., Pegylated Leptin Antagonist Is a Potent Orexigenic Agent: Preparation and Mechanism of Activity, Endocrinology, 2009, 150(7), 3083–3091.
- http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000420/WC500025941.pdf.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization directly from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.