PEG products with additional MW may be made to order, please contact us for details
Linear Methoxy PEG Raw Materials
$775.00 – $1,150.00
Description
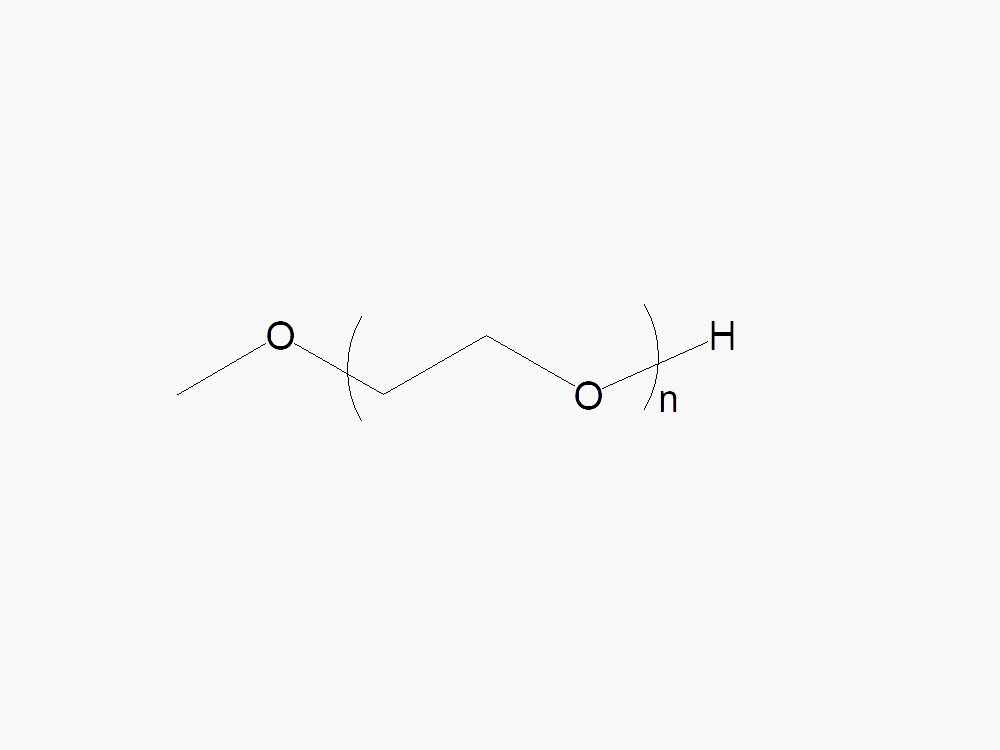
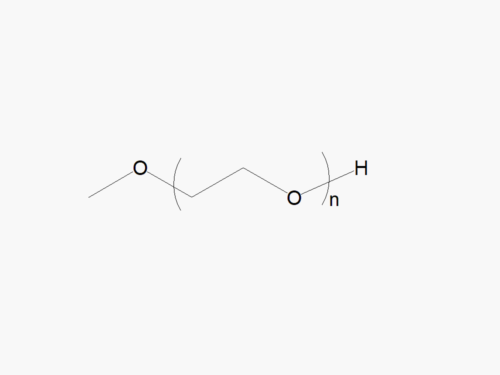
JenKem Technology’s PEG linear raw materials are high quality PEGs with very low polydispersity, and low or no diol content, suitable for further derivatization into PEG derivatives. Linear methoxy PEG raw materials are provided as bulk orders (100g-500g) with molecular weights of 2kDa, 5kDa, 10kDa, 20kDa, 30kDa, and 40kDa.
JenKem Technology also provides PEG GPC Calibration Standard kits for linear methoxy PEGs with MW of 2kDa, 5kDa, 10kDa, 20kDa and 40kDa.
Click here to download the MSDS
| PEG PRODUCT | AVAILABLE MW | MAIN PEAK FRACTION BY GPC | POLYDISPERSITY BY GPC |
| M-PEG-OH | M-PEG-2000 (2kDa), M-PEG-5000 (5kDa), M-PEG-10K (10kDa), M-PEG-20K (20kDa), M-PEG-30K (30kDa), M-PEG-40K (40kDa) | ≥ 95% | ≤ 1.05-1.10 |
Our GMP grade methoxy PEG derivatives are manufactured with high purity mPEG raw materials. Please click on this link for more details.
References:
- Bunk, C., et al., Amphiphilic tetra-PCL-b-PEG star block copolymers using benzoxazinone-based linking groups, Polymer Chemistry, 4(16), 2023.
- Robert, J., et al., Coiled-coil peptide-based assembly of a plasmonic core-satellite polymer-metal nanocomposite as an efficient photothermal agent for drug delivery applications, Journal of Colloid and Interface Science, 641, 2023.
- Wong, K.H., et al., Linear-like polypeptide-based micelle with pH-sensitive detachable PEG to deliver dimeric camptothecin for cancer therapy, Asian Journal of Pharmaceutical Sciences, V. 18(1), 2023.
- Ferrado, J. B., et al., PEGylation of genistein-loaded bovine serum albumin nanoparticles and its effect on in vitro cell viability and genotoxicity properties, Colloids and Surfaces B: Biointerfaces, V. 222, 2023.
- Robert, J, et al., Coiled-Coil Peptide-Based Assembly of a Plasmonic Core-Satellite Polymer-Metal Nanocomposite as an Efficient Photothermal Agent, NANOTODAY-D-22-00764, 2022.
- Welzen, PL, et al., Reversibly self‐assembled pH‐responsive PEG‐p (CL‐g‐TMC) polymersomes. Journal of Polymer Science. 2021.
- Yang, Z., et al., Defeating relapsed and refractory malignancies through a nano-enabled mitochondria-mediated respiratory inhibition and damage pathway, Biomaterials, 2020, V. 229.
- Jones, S.K., et al., Correlating quantitative tumor accumulation and gene knockdown using SPECT/CT and bioluminescence imaging within an orthotopic ovarian cancer model, Biomaterials, 2018, V. 178, P. 183-192.
- Long, T., et al., Covalent Immobilization of Enoxacin onto Titanium Implant Surfaces for Inhibiting Multiple Bacterial Species Infection and In Vivo Methicillin-Resistant Staphylococcus aureus Infection Prophylaxis. Antimicrobial Agents and Chemotherapy, 2017, 61(1), e01766-16.
- Mu, J., et al., Highly stable and biocompatible W18O49@PEG-PCL hybrid nanospheres combining CT imaging and cancer photothermal therapy, RSC Advances, 2017, 7(18):10692-9.
- Wang Q, et al., Targeting NF-kB signaling with polymeric hybrid micelles that co-deliver siRNA and dexamethasone for arthritis therapy, Biomaterials, 2017.
- Alqahtani, M.S., et al., Food Protein Based Core–Shell Nanocarriers for Oral Drug Delivery: Effect of Shell Composition on in Vitro and in Vivo Functional Performance of Zein Nanocarriers. Molecular pharmaceutics, 2017, 14(3):757-69.
- Jackson, M.A., et al., Zwitterionic Nanocarrier Surface Chemistry Improves siRNA Tumor Delivery and Silencing Activity Relative to Polyethylene Glycol. ACS Nano, 2017.
- Del Pino, J.A., et al., Delivery of HSP90 Inhibitor Using Water Soluble Polymeric Conjugates with High Drug Payload. Pharmaceutical research, 2017, 1-4.
- Ren, S., et al., Hypotoxic and Rapidly Metabolic PEG-PCL-C3-ICG Nanoparticles for Fluorescence-Guided Photothermal/Photodynamic Therapy against OSCC, ACS applied materials & interfaces, 2017, 9(37), 31509-18.
- Wang, Y., et al., Quantum-Dot-Based Theranostic Micelles Conjugated with an Anti-EGFR Nanobody for Triple-Negative Breast Cancer Therapy, ACS applied materials & interfaces, 2017, 9(36), 30297-305.
- Chen, G., et al., KE108-conjugated unimolecular micelles loaded with a novel HDAC inhibitor thailandepsin-A for targeted neuroendocrine cancer therapy, Biomaterials, 2016, 97, p. 22-33.
- Jain, S., et al., Estradiol functionalized multi-walled carbon nanotubes as renovated strategy for efficient gene delivery, RSC Advances, 2016, 6(13), 10792-801.
- Jaskula-Sztul, R., et al., Thailandepsin A-loaded and octreotide-functionalized unimolecular micelles for targeted neuroendocrine cancer therapy, Biomaterials, 2016, 91:1-0.
- Wang, Q., et al., Targeted delivery of low-dose dexamethasone using PCL–PEG micelles for effective treatment of rheumatoid arthritis. Journal of Controlled Release, 2016, 230, 64-72.
- Guastavino, J.F., et al., Simple Synthesis of Aldehyde and Carboxylic Acid Terminated Methoxypoly (ethylene glycol), Macromolecular Chemistry and Physics, 2016.
- Ron, T., et al., Ultralow Friction with Hydrophilic Polymer Brushes in Water as Segregated from Silicone Matrix, Adv. Mater. Interfaces, 2015.
- Miteva, M., et al., Tuning PEGylation of mixed micelles to overcome intracellular and systemic siRNA delivery barriers, Biomaterials, 38, 2015, p: 97-107.
- Fan, Z., et al., Adding Vitamin E-TPGS to the Formulation of Genexol-PM: Specially Mixed Micelles Improve Drug-Loading Ability and Cytotoxicity against Multidrug-Resistant Tumors Significantly, PLOS ONE, 2015.
- Campbell, M.L., et al., Target-Specific Capture of Environmentally Relevant Gaseous Aldehydes and Carboxylic Acids with Functional Nanoparticles, Chem. Eur. J., 2015, 21, 14834–14842.
- He, X., et al., pH-Responsive Wormlike Micelles with Sequential Metastasis Targeting Inhibit Lung Metastasis of Breast Cancer, Adv. Healthcare Mater., 2015, 2192-2659.
- Gonzalez, M., et al., Novel reactive PEG for amino group conjugation, RSC Adv., 2015, 5, 14002-14009.
- Gu, D., et al., Amphiphilic core cross-linked star polymers as water-soluble, biocompatible and biodegradable unimolecular carriers for hydrophobic drugs, Polym. Chem., 2015, 6, 6475-6487.
- He, X., et al., Tumor-Penetrating Nanotherapeutics Loading a Near-Infrared Probe Inhibit Growth and Metastasis of Breast Cancer, Adv. Funct. Mater., 2015, 25, 2831–2839.
- Peng, W., et al., Oral delivery of capsaicin using MPEG-PCL nanoparticles, Acta Pharmacologica Sinica, 2015, 36, 139–148.
- Chen, J., et al., Reducible polyamidoamine-magnetic iron oxide self-assembled nanoparticles for doxorubicin delivery. Biomaterials, 2014. 35(4): p. 1240-1248.
- Chen, J., et al., A Facile Strategy for In Situ Controlled Delivery of Doxorubicin with a pH-Sensitive Injectable Hydrogel, Nano LIFE, 2014, 04:03.
- Mattix, B.,et al., Accelerated Iron Oxide Nanoparticle Degradation Mediated by Polyester Encapsulation within Cellular Spheroids, Adv. Funct. Mater., 2014, 24: 800–807.
- Mattix, B., et al., Effects of polymeric nanoparticle surface properties on interaction with brain tumor environment, Nano Life, 2013, 03:04.
- Xu, W., Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer, Biomaterials, 2013, 34(21), p: 5244-5253.
- Ron, T., et al., Adsorption and Aqueous Lubricating Properties of Charged and Neutral Amphiphilic Diblock Copolymers at a Compliant, Hydrophobic Interface, Langmuir, 2013, 29(25), p: 7782–7792.
- Fasinu, P., et al., Flavonoids and polymer derivatives as CYP3A4 inhibitors for improved oral drug bioavailability, Journal of Pharmaceutical Sciences, 2013, 102(2): 541-555.
- Chen, J., et al., Photo-cross-linked and pH-Sensitive Biodegradable Micelles for Doxorubicin Delivery, ACS Applied Materials & Interfaces, 2013, 5 (8), 3108-3117.
- Qian, Y., et al., PEGylated poly (2-(dimethylamino) ethyl methacrylate)/DNA polyplex micelles decorated with phage-displayed TGN peptide for brain-targeted gene delivery, Biomaterials, 2013, 34.8, 2117-2129.
- Das, M., et al., Intranuclear Drug Delivery and Effective in Vivo Cancer Therapy via Estradiol–PEG-Appended Multiwalled Carbon Nanotubes, Mol. Pharmaceutics, 2013, 10 (9), p. 3404–3416.
- Xu, W., et al., Octreotide-functionalized and resveratrol-loaded unimolecular micelles for targeted neuroendocrine cancer therapy, Nanoscale, 2013, 5.20 : 9924-9933.
- Gonzalez, M., et al., New method for the synthesis and purification of branched mPEG2lys, Reactive and Functional Polymers, 2012, 72(1), p: 107-113.
- Chen, J., et al., Reducible self-assembled micelles for enhanced intracellular delivery of doxorubicin, J. Mater. Chem., 2012, 22, 7121-7129.
- Jiang, X., et al., PEGylated poly(trimethylene carbonate) nanoparticles loaded with paclitaxel for the treatment of advanced glioma: In vitro and in vivo evaluation, International Journal of Pharmaceutics, 2011, 420(2),p: 385–394.
- Chen, J., et al., pH and Reduction Dual-Sensitive Copolymeric Micelles for Intracellular Doxorubicin Delivery, Biomacromolecules, 2011, 12(10), 3601-3611.
- Jiang, X., et al., Self-aggregated pegylated poly (trimethylene carbonate) nanoparticles decorated with c(RGDyK) peptide for targeted paclitaxel delivery to integrin-rich tumors, Biomaterials, 2011, 32(35), p:9457–9469.
- Qian, H., et al., A Strategy for Control of “Random” Copolymerization of Lactide and Glycolide: Application to Synthesis of PEG-b-PLGA Block Polymers Having Narrow Dispersity, Macromolecules, 2011, 44, 7132–7140.
- Xu, J., et al., Cytocompatible poly(ethylene glycol)-co-polycarbonate hydrogels cross-linked by copper-free, strain-promoted click chemistry, Chem Asian J, 2011, 6(10): 2730-2737.
- Aryal, S., et al., Polymer−Cisplatin Conjugate Nanoparticles for Acid-Responsive Drug Delivery, ACS Nano, 2010, 4(1), 251-258.
- Yan, H., et al., Electroless plating of silver nanoparticles on porous silicon for laser desorption/ionization mass spectrometry, International Journal of Mass Spectrometry, 2009, 281:1–2, P. 1-7.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.