
JenKem Technology manufactures PEG raw materials with high purity and low polydispersity in 5kg, 15kg, 20kg, and 100kg batch sizes. These PEG raw materials are used for JenKem Technology’s manufacture of high quality PEG derivatives. JenKem Technology’s in-house PEG production is back-integrated to polymerization from ethylene oxide.
Linear PEG Raw Materials
JenKem Technology provides high quality linear methoxy and benzyl polyethylene glycol raw materials with high purity, low polydispersity, and low to no diol content. These PEG raw materials are end-capped with a methoxy group or benzyl group. Linear PEG raw materials are provided online as bulk orders of 100g-500g, with the molecular weights of 2kDa, 5kDa, 10kDa, 20kDa, 30kDa, and 40kDa.
Starting 2020, JenKem Technology will transition to use High Purity (> 99%) mPEG Raw Materials to manufacture GMP grade Methoxy PEG Derivatives.
JenKem Technology’s mPEG alcohols are suitable for further derivatization into PEG derivatives.
JenKem Technology also provides a Methoxy PEG GPC Calibration Standards kit (STDMPEG) containing linear methoxy PEGs with MW of 2kDa, 5kDa, 10kDa, 20kDa and 40kDa.
Multi-Arm PEG Raw Materials
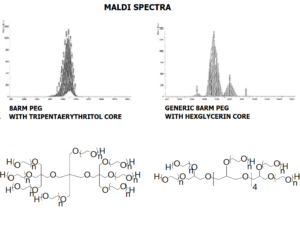
JenKem Technology provides 4ARM and 8ARM PEG raw materials in 100g-500g online order size.
8ARM-PEG-OH (8arm PEG hydroxyl) is prepared either by ethoxylation of tripentaerythritol (TP core), or hexaglycerine. 8ARM(TP)-PEGs with tripentaerythritol core have a higher purity as evidenced by MALDI compared to the generic 8ARM-PEGs with a hexaglycerin core. 6ARM-PEG-OH (6arm PEG hydroxyl) is prepared by ethoxylation of dipentaerythritol.
4ARM-PEG-OH (4arm PEG hydroxyl) is prepared by ethoxylation of pentaerythritol. JenKem Technology also provides a 4ARM PEG GPC Calibration Standards kit (STD4ARMPEG) that includes 200 mg each of 4ARM-PEG-2000, 4ARM-PEG-5000, 4ARM-PEG-10K, 4ARM-PEG-20K, and 4ARM-PEG-40K.
JenKem Technology reports the total molecular weight of multi-arm PEG raw materials as the sum of the molecular weights of all the arms. The number of ethylene oxide units in the PEG chain may not be equal for all arms.
JenKem Technology’s PEG raw materials with molecular weights and branching not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability of custom polyethylene glycols.
For global distribution, please visit link. Click the buttons below to order directly from JenKem Technology:
| PEG PRODUCT | MOLECULAR WEIGHT | MAIN PEAK FRACTION BY GPC | POLYDISPERSITY BY GPC | REFERENCES |
|---|---|---|---|---|
| 2kDa, 5kDa, 10kDa, 20kDa, 30kDa, 40kDa | ≥ 95% | ≤ 1.05-1.10 | [2-4] | |
| 2kDa, 5kDa | ≥ 95% | ≤ 1.05 | [5] | |
| 2kDa, 5kDa, 15kDa, 10kDa, 20kDa | ≥ 95% | ≤ 1.05 | [6-19, 32] | |
| 15kDa, 30kDa | ≥ 95% | ≤ 1.08 | [20, 32] | |
| 10kDa, 15kDa, 20kDa, 40kDa | ≥ 95%; > 90% for MW 40,000 | ≤ 1.08 | [21-25, 32] | |
| 10kDa, 15kDa, 20kDa, 40kDa | ≥ 95% | ≤ 1.12 | [26-32] |
PEG Raw Materials for discrete PEG derivatives with purity ≥ 95% are also available from small through large commercial scale. Please visit the following page to view a few examples of monodisperse PEG raw materials and contact us if the structure you need is not listed on the link:
References:
- Hutanu, D., et al., Recent Applications of Polyethylene Glycols (PEGs) and PEG Derivatives. Mod Chem appl, 2014, 2(132).
- Yang, Z., et al., Defeating relapsed and refractory malignancies through a nano-enabled mitochondria-mediated respiratory inhibition and damage pathway, Biomaterials, 2020, V. 229.
- Jackson, M.A., et al., Zwitterionic Nanocarrier Surface Chemistry Improves siRNA Tumor Delivery and Silencing Activity Relative to Polyethylene Glycol, ACS Nano, 2017.
- Mu, J., et al., Highly stable and biocompatible W18O49@PEG-PCL hybrid nanospheres combining CT imaging and cancer photothermal therapy, RSC Advances, 2017, 7(18).
- Mueller, C., et al., Noncovalent PEGylation: Different Effects of Dansyl-, l-Tryptophan–, Phenylbutylamino-, Benzyl- and Cholesteryl-PEGs on the Aggregation of Salmon Calcitonin and Lysozyme, Journal of Pharmaceutical Sciences, 2012, V. 101:6, p. 1995 – 2008.
- Lee, S., et al., Inflammatory responses of macrophage-like RAW264.7 cells in a 3D hydrogel matrix to ultrasonicated schizophyllan, Carbohydrate Polymers, 2020, V. 229.
- Kwak, H., et al., Colorimetric assay of tyrosinase inhibition using melanocyte laden hydrogel fabricated by digital light processing printing, Journal of Industrial and Engineering Chemistry, 2020, V. 84, P. 252-259.
- Ma, H., et al., Bioorthogonal click chemistries enable simultaneous spatial patterning of multiple proteins to probe synergistic protein effects on fibroblast function, Biomaterials, 2020, 255:120205.
- Eles, J.R., et al., Meningeal inflammatory response and fibrous tissue remodeling around intracortical implants: An in vivo two-photon imaging study, Biomaterials, 2019, 195, P. 111-12.
- Xin, S., et al., Clickable PEG Hydrogel Microspheres as Building Blocks for 3D Bioprinting, Biomaterials science, 2019.
- Tae, H., et al., β-glucan hybridized poly (ethylene glycol) microgels for macrophage-targeted protein delivery, Journal of Industrial and Engineering Chemistry, 2019.
- Jones, C.E., et al., Stromal PTEN Regulates Extracellular Matrix Organization in the Mammary Gland, Neoplasia, 2019, 21(1):132-45.
- Shagan, A., et al., Near-Infrared Light Induced Phase Transition of Biodegradable Composites for On-Demand Healing and Drug Release, ACS applied materials & interfaces, 2018, 10(4), p.4131-4139.
- Carthew, J., et al., Polyethylene glycol–gelatin hydrogels with tuneable stiffness prepared by horseradish peroxidase-activated tetrazine–norbornene ligation, Journal of Materials Chemistry B, 2018, 6:9.
- Croitoru-Sadger, T., et al., Two-component cross-linkable gels for fabrication of solid oral dosage forms, Journal of Controlled Release, 2019.
- Khodamoradi, M., et al., An electro-conductive hybrid scaffold as an artificial Bruch’s membrane, Materials Science and Engineering: C, 2021, V. 126.
- Machado Cruz, R., et al., Impact of polyethylene glycol polymers on the physicochemical properties and mucoadhesivity of itraconazole nanoparticles, European Journal of Pharmaceutics and Biopharmaceutics, 2019, 144, p. 57-67.
- Kwak, H., et al., Formation of a keratin layer with silk fibroin-polyethylene glycol composite hydrogel fabricated by digital light processing 3D printing, Journal of Industrial and Engineering Chemistry, 2018.
- Saghiri, M.A., et al., Hydrogel Arrays and Choroidal Neovascularization Models for Evaluation of Angiogenic Activity of Vital Pulp Therapy Biomaterials, Journal of endodontics, 2018, 44(5), pp.773-779.
- Richbourg NR, et al., Solute diffusion and partitioning in multi-arm poly (ethylene glycol) hydrogels. Journal of Materials Chemistry B. 2023; 11(2):377-88.
- Gangolphe, L., et al., Degradable multi (aryl azide) star copolymer as universal photo-crosslinker for elastomeric scaffolds, Materials Today Chemistry, 2019, 12:209-21.
- Peuler, K. et al., Clickable modular polysaccharide nanoparticles for selective cell-targeting. Carbohydrate Polymers. 2020; 234:115901.
- Graf, M., et al., Hydrogel microspheres evading alveolar macrophages for sustained pulmonary protein delivery, International Journal of Pharmaceutics, 2019, 566, p.652-661.
- Chu, S., et al., Cell encapsulation spatially alters crosslink density of poly(ethylene glycol) hydrogels formed from free-radical polymerizations, Acta Biomaterialia, 2020, 109, p. 37-50.
- Wang, C., et al., A comparative study of brain tumor cells from different age and anatomical locations using 3D biomimetic hydrogels. Acta Biomaterialia, 2020.
- Li, Y., et al., Matrix metalloproteinase (MMP)-degradable tissue engineered periosteum coordinates allograft healing via early stage recruitment and support of host neurovasculature, Biomaterials, 2021, V. 268.
- Ziegler, C. E., et al., A novel anhydrous preparation of PEG hydrogels enables high drug loading with biologics for controlled release applications, European Polymer Journal, 2021, V. 147.
- Crocini, C., et al., Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness, Progress in Biophysics and Molecular Biology, 2019.
- Lee, J., et al., Micromechanical property mismatch between pericellular and extracellular matrices regulates stem cell articular and hypertrophic chondrogenesis, Matter 2023, 6.2: 475-492.
- Wang, C., et al., Mimicking brain tumor-vasculature microanatomical architecture via co-culture of brain tumor and endothelial cells in 3D hydrogels, Biomaterials, 2019.
- Wang, R., et al., Enzymatic co-crosslinking of star-shaped poly (ethylene glycol) tyramine and hyaluronic acid tyramine conjugates provides elastic biocompatible and biodegradable hydrogels, Bioactive Materials, 2023.
- Wang, J., et al., Quantitation of polyethylene glycol by size exclusion chromatography with charged aerosol, differential refractive index, and multi-angle light scattering detectors, Journal of Pharmaceutical and Biomedical Analysis, 238, 2024.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.
