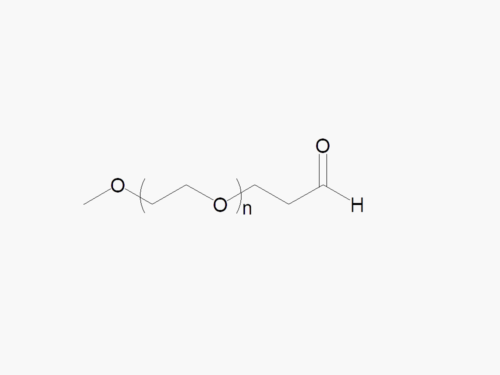
PEG products with additional MW may be made to order, please contact us for details
Methoxy PEG Maleimide
$130.00 – $5,200.00
Description
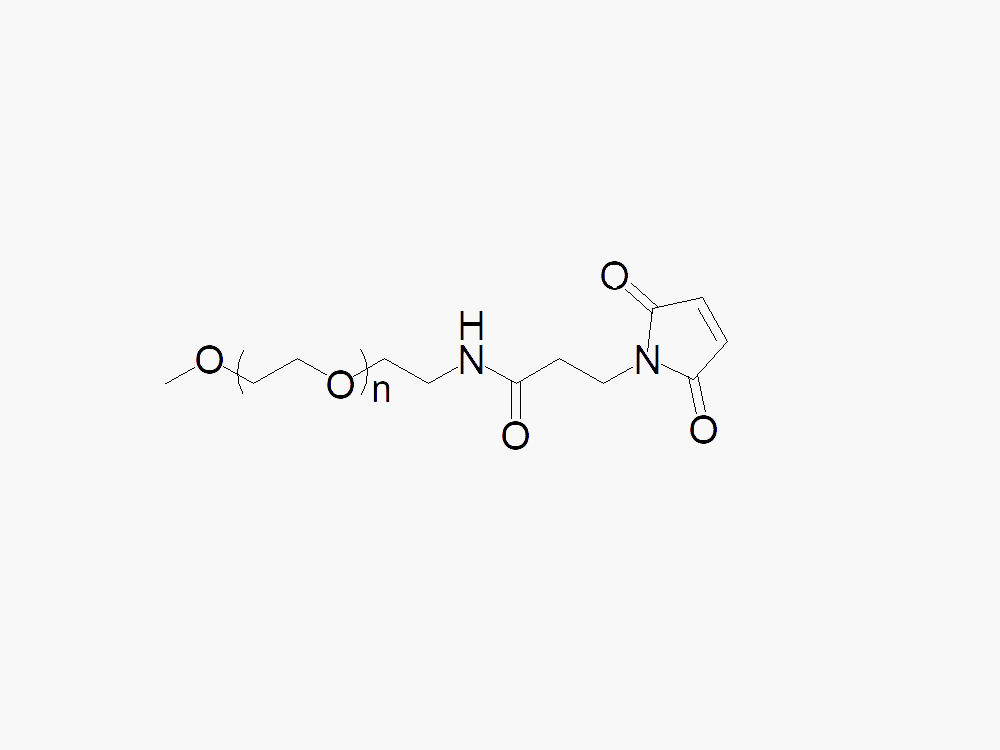
Methoxy PEG Maleimide with superior quality specification of ≥95% Substitution.
Methoxy PEG Maleimide (mPEG-MAL) from JenKem Technology is is a thiol reactive PEG derivative selective for thiol groups on cystein side chains. Methoxy PEG Maleimide undergoes thiol PEGylation reactions with thiol-containing molecules at pH 5.0-6.5. JenKem Technology offers Methoxy PEG Maleimide with MW 2kDa (M-MAL-2000), MW 5kDa (M-MAL-5000), MW 10kDa (M-MAL-10K), MW 20kDa (M-MAL-20K), MW 30kDa (M-MAL-30K) and MW 40kDa (M-MAL-40K) in 1g and 10g packing sizes.
Different MW of Methoxy PEG Maleimide products may be available by custom synthesis, please email us at tech@jenkemusa.com for details on custom PEGs. JenKem Technology provides repackaging services for an additional fee, please contact us if you require a different package size than our catalog selection.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Lee, H. S., et al., Hypoxia-alleviating hemoglobin nanoclusters for sensitizing chemo-photodynamic therapy of cervical cancer, Chemical Engineering Journal, V. 457, 2023.
- Umeshappa, C. S., et al., Liver-specific T regulatory type-1 cells program local neutrophils to suppress hepatic autoimmunity via CRAMP, Cell Reports, 2021, V. 34(13).
- Yang Y, et al., Extremely short bioavailability and fast pharmacodynamic effects of pMHC-based nanomedicines. Journal of Controlled Release. 2021, 338:557-70.
- Qi, F., at al., Conjugation of staphylokinase with the arabinogalactan-PEG conjugate: Study on the immunogenicity, in vitro bioactivity and pharmacokinetics, International Journal of Biological Macromolecules, 2019, V. 131, P. 896-904.
- Peng, G., et al., N-terminal site-specific PEGylation enhances the circulation half-life of Thymosin alpha 1, Journal of Drug Delivery Science and Technology, 2019, 49:405-12.
- Shen, L., et al., Facile synthesis of organosilica-capped mesoporous silica nanocarriers with selective redox-triggered drug release properties for safe tumor chemotherapy, Biomaterials science, 2019.
- Kaminskas, L.M., et al., A 30 kDa polyethylene glycol-enfuvirtide complex enhances the exposure of enfuvirtide in lymphatic viral reservoirs in rats, European Journal of Pharmaceutics and Biopharmaceutics, 2019.
- Lin, S.Y., et al., Multifunctional PEGylated Albumin/IR780/Iron Oxide Nanocomplexes for Cancer Photothermal Therapy and MR Imaging, Nanotheranostics, 2018, 2(2), p.106.
- DeGroot, A.C., et al., Entropic control of receptor recycling using engineered ligands, Biophysical journal, 2018, 114(6), p.1377-1388.
- Li, X., et al., Amphiphilic dendrimer engineered nanocarrier systems for co-delivery of siRNA and paclitaxel to matrix metalloproteinase-rich tumors for synergistic therapy, NPG Asia Materials, 2018, p.1.
- Pereira Gomes, C., et al., Fine tuning neuronal targeting of nanoparticles by adjusting the ligand grafting density and combining PEG spacers of different length, Acta Biomaterialia, 2018, V. 78, P. 247-259.
- Chang, X., et al., Conjugation of PEG-hexadecane markedly increases the immunogenicity of pneumococcal polysaccharide conjugate vaccine, Vaccine, 2017, 35(13):1698-704.
- Wan, X., et al., Effect of protein immunogenicity and PEG size and branching on the anti-PEG immune response to PEGylated proteins, Process Biochemistry, 2017, 52:183-91.
- Wang, Y., et al., A PEGylated bovine hemoglobin as a potent hemoglobin‐based oxygen carrier, Biotechnology progress, 2017, 33(1):252-60.
- Zhao, Y.Z., et al., PEGylation with the thiosuccinimido butylamine linker significantly increases the stability of haloalkane dehalogenase DhaA, Journal of Biotechnology, 2017.
- Xu, L., et al., Oxidized catechol-derived poly (ethylene glycol) for thiol-specific conjugation. Reactive and Functional Polymers, 2017.
- Zhang, C., et al., Development of long-acting ciliary neurotrophic factor by site-specific conjugation with different-sized polyethylene glycols and transferrin, International Journal of Pharmaceutics, 2017.
- Chen, Y., et al., Prodrug-Like, PEGylated Protein Toxin Trichosanthin for Reversal of Chemoresistance. Molecular Pharmaceutics, 2017, 14(5):1429-38.
- Chen, Y., et al., Intein-mediated site-specific synthesis of tumor-targeting protein delivery system: Turning PEG dilemma into prodrug-like feature, Biomaterials, 2017, V. 116, P. 57-68.
- Zhang, C., et al., Development of long-acting ciliary neurotrophic factor by site-specific conjugation with different-sized polyethylene glycols and transferrin, International Journal of Pharmaceutics, 2017, V. 529, P. 275-284.
- Chan, L.J., et al., Conjugation of 10 kDa linear PEG onto trastuzumab Fab’is sufficient to significantly enhance lymphatic exposure while preserving in vitro biological activity, Molecular Pharmaceutics, 2016.
- Sawhney, P., et al., PEGylation of Truncated Streptokinase Leads to Formulation of a Useful Drug with Ameliorated Attributes. PloS one, 2016, 11(5):e0155831.
- Turaga, R.C., et al., Rational design of a protein that binds integrin [alpha] v [beta] 3 outside the ligand binding site. Nature communications, 2016, 7.
- Gao, Y., et al., Multifunctional gold nanostar-based nanocomposite: Synthesis and application for noninvasive MR-SERS imaging-guided photothermal ablation, Biomaterials, 2015, 60: 31-41.
- Gupta, M.K., et al., Oligoproline-derived nanocarrier for dual stimuli-responsive gene delivery, J. Mater. Chem. B, 2015, 3, 7271-7280.
- Li, X., et al., D-SP5 Peptide-Modified Highly Branched Polyethylenimine for Gene Therapy of Gastric Adenocarcinoma, Bioconjugate Chemistry, 2015, 26 (8), 1494-1503.
- Brown, A.C., et al., Molecular interference of fibrin’s divalent polymerization mechanism enables modulation of multiscale material properties, Biomaterials, 2015, 49, P. 27-36.
- Wang, J., et al., Size- and surface chemistry-dependent pharmacokinetics and tumor accumulation of engineered gold nanoparticles after intravenous administration, Metallomics, 2015, 7, 516-524.
- Ren, H., et al., Peptide GE11–Polyethylene Glycol–Polyethylenimine for targeted gene delivery in laryngeal cancer, Medical Oncology, 2015, 32:185.
- Zhang, T., et al., Moderate PEGylation of the carrier protein improves the polysaccharide-specific immunogenicity of meningococcal group A polysaccharide conjugate vaccine, Vaccine, 2015, 33:28, P. 3208-3214.
- Hadadian, S., et al., Stability and biological activity evaluations of PEGylated human basic fibroblast growth factor, Advanced biomedical research, 2015, 4.
- Wang, J., et al., Retro-Inverso CendR Peptide-Mediated Polyethyleneimine for Intracranial Glioblastoma-Targeting Gene Therapy, Bioconjugate Chem., 2014, 25(2) p:414–423.
- Yu, W., et al., PEGylated recombinant human interferon-ω as a long-acting antiviral agent: Structure, antiviral activity and pharmacokinetics. Antiviral Research, 2014, 108: p. 142-147.
- Yang, Y.J., et al., Production and characterization of a fusion peptide derived from the rabies virus glycoprotein (RVG29), Protein Expression and Purification, 2014, 104, p: 7-13.
- Zhu, M., et al., Adsorption of polyethylene-glycolated bovine serum albumin on macroporous and polymer-grafted anion exchangers, Journal of Chromatography A, 2014, V. 1326, p. 29-38.
- Kharkar, P.M., et al., Dually degradable click hydrogels for controlled degradation and protein release, J. Mater. Chem. B, 2014, 2, 5511-5521.
- Hadadian, S., et al., Chemoselective PEGylation of cysteine analogs of human basic fibroblast growth factor (hbFGF) – design and expression, AJOL, 2014, 13:10.
- Yang, J., et al., Targeted delivery of the RGD-labeled biodegradable polymersomes loaded with the hydrophilic drug oxymatrine on cultured hepatic stellate cells and liver fibrosis in rats, European Journal of Pharmaceutical Sciences, 2014, 52:180-90.
- Sakoh-Nakatogawa, M., et al., Atg12–Atg5 conjugate enhances E2 activity of Atg3by rearranging its catalytic site, Nature Structural & Molecular Biology, 2013, 20, p: 433–439.
- Williams, C., et al, A disulphide bond in the E2 enzyme Pex4p modulates ubiquitin-conjugating activity, Scientific Reports, 2013, 3 : 2212
- Mu Q, et al., Molecular Insight into the Steric Shielding Effect of PEG on the Conjugated Staphylokinase: Biochemical Characterization and Molecular Dynamics Simulation, PLoS ONE, 2013, 8(7): e68559.
- Gao, M., et al., Development of a C-Terminal Site-Specific PEGylated Analog of GLP-1 with Improved Anti-Diabetic Effects in Diabetic Mice, Drug Development Research, 2013, 74(3): 186-193.
- Qian, Y., et al., PEGylated poly (2-(dimethylamino) ethyl methacrylate)/DNA polyplex micelles decorated with phage-displayed TGN peptide for brain-targeted gene delivery, Biomaterials, 2013, 34.8: 2117-2129.
- Gao. M., et al., PEGylation-aided refolding of globular adiponectin. World Journal of Microbiology and Biotechnology, 2013, 29(8):1525-30.
- Wang, J., et al., Targeted gene delivery to glioblastoma using a C-end rule RGERPPR peptide-functionalised polyethylenimine complex. International journal of pharmaceutics, 2013, 458(1):48-56.
- Szerencsei, R. T., et al., The topology of the C-terminal sections of the NCX1 Na+/Ca2+ exchanger and the NCKX2 Na+/Ca2+-K+ exchanger, Channels, 2013, 7.2 : 109-114.
- Sun, C., et al., Bifunctional PEGylated exenatide-amylinomimetic hybrids to treat metabolic disorders: an example of long-acting dual hormonal therapeutics, Journal of medicinal chemistry, 2013, 56.22 : 9328-9341.
- Wu, H., et al., A novel small Odorranalectin-bearing cubosomes: Preparation, brain delivery and pharmacodynamic study on amyloid-β25–35-treated rats following intranasal administration, European Journal of Pharmaceutics and Biopharmaceutics, 2012, 80(2), p: 368-378.
- Liu, S.. et al., Mono-PEGylation of ribonuclease A: High PEGylation efficiency by thiolation with small molecular weight reagent, Process Biochemistry, 2012, 47(9), p: 1364-1370.
- Tang, L., et al., Separation and detection of bis-maleimide-polyethylene glycol and mono-maleimide-polyethylene glycol by reversed-phase high pressure liquid chromatography, Journal of Chromatography A, 2012, 1246, p: 117–122.
- Zhan, C., et al., Co-delivery of TRAIL gene enhances the anti-glioblastoma effect of paclitaxel in vitro and in vivo, Journal of Controlled Release, 2012, 160:3, P. 630-636.
- Xin, H., et al., Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma, Biomaterials, 2011, 32(18), p: 4293-4305.
- Kawai, M., et al., The Heparin-binding Domain of IGFBP-2 Has Insulin-like Growth Factor Binding-independent Biologic Activity in the Growing Skeleton, The Journal of Biological Chemistry, 2011, 286, p:14670-14680.
- Stabenfeldt, S. E., et al., A new direction for anticoagulants: Inhibiting fibrin assembly with PEGylated fibrin knob mimics, Biotechnology and bioengineering, 2011, 108(10): 2424-2433.
- Rolf J. Verheul, R.J, et al., Covalently stabilized trimethyl chitosan-hyaluronic acid nanoparticles for nasal and intradermal vaccination, Journal of Controlled Release, 2011, V. 156:1, P. 46-52.
- Kaili Hu, K., et al., Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinson’s disease, International Journal of Pharmaceutics, 2011, 415:1–2, 30, P. 273-283.
- Resch, G., P. Moreillon, and V.A. Fischetti, PEGylating a bacteriophage endolysin inhibits its bactericidal activity. AMB Express, 2011, 1(29): p. 1-5.
- Zhan, C., et al., Cyclic RGD-poly(ethylene glycol)-polyethyleneimine is more suitable for glioblastoma targeting gene transfer in vivo, Journal of Drug Targeting, 2011, 19:7.
- Lamppa, J.W, et al., Genetically Engineered Alginate Lyase-PEG Conjugates Exhibit Enhanced Catalytic Function and Reduced Immunoreactivity. II RR, ed. PLoS ONE, 2011, 6(2):e17042.
- Barth, B.M., et al., Bioconjugation of Calcium Phosphosilicate Composite Nanoparticles for Selective Targeting of Human Breast and Pancreatic Cancers In Vivo, ACS Nano, 2010, 4(3), p. 1279–1287.
- Gao, M., et al., Expression, Purification, and C-terminal Site-Specific PEGylation of Cysteine-Mutated Glucagon-Like Peptide-1, Applied Biochemistry and Biotechnology, 2010, V. 162:1, p. 155-165.
- De Jaco, A., et al., Folding anomalies of neuroligin3 caused by a mutation in the α/β-hydrolase fold domain, Chemico-Biological Interactions, 2010, V. 187:1–3, P. 56-58.
- Park, J.-B., et al., PEGylation of bacterial cocaine esterase for protection against protease digestion and immunogenicity. Journal of Controlled Release, 2010, 142(2), p. 174-179.
- Pacchiana, G., et al., Monovalency Unleashes the Full Therapeutic Potential of the DN-30 Anti-Met Antibody, The Journal of Biological Chemistry, 2010, 285, 36149-36157.
- Sharma, R., et al., Bioconjugation of Calcium Phosphate Nanoparticles for Selective Targeting of Human Breast and Pancreatic Cancers In Vivo, ACS nano, 2010, 4(3):1279-1287.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom polymer synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.