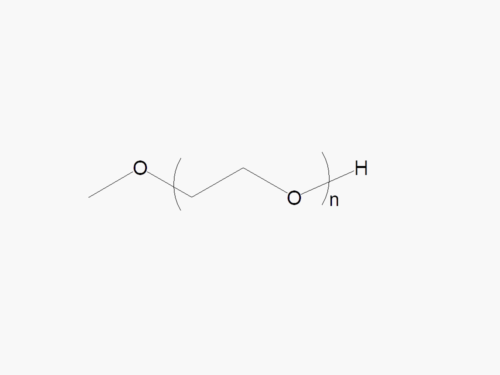
PEG products with additional MW may be made to order, please contact us for details
Methoxy PEG Thiol
$120.00 – $1,360.00
Description
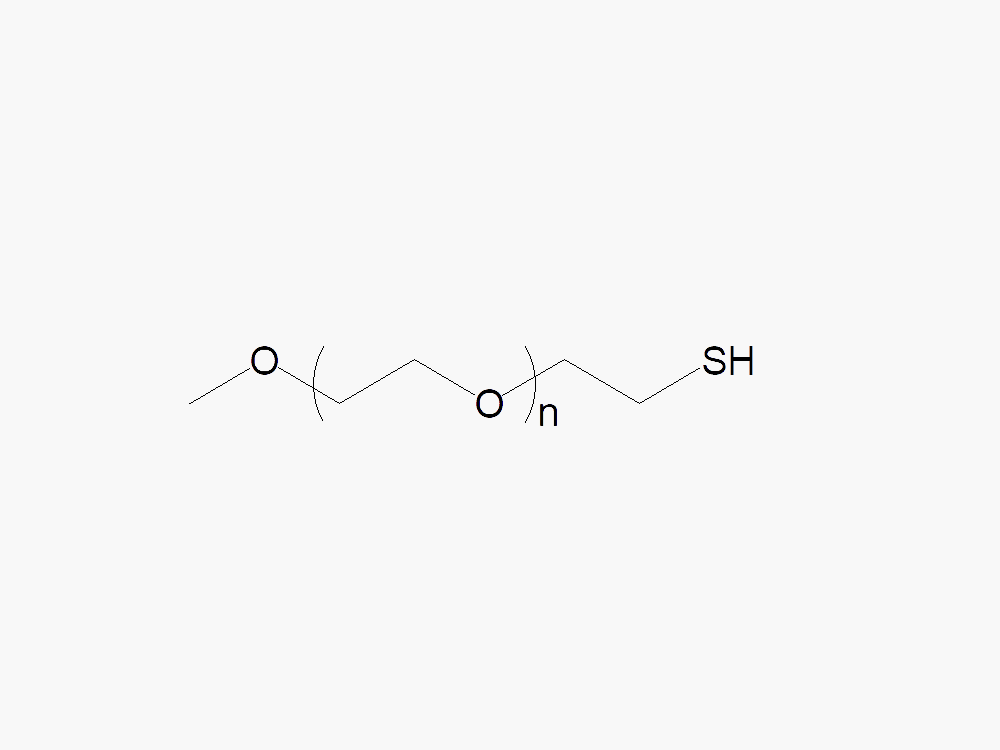
Methoxy PEG Thiol with superior quality specification of ≥95% Substitution.
Methoxy PEG Thiol is a high quality activated PEG product for thiol pegylation. Methoxy PEG Thiol PEGylates the thiol groups on cysteine side chains under mild reaction conditions. JenKem Technology offers Methoxy PEG Thiol with MW 5kDa (M-SH-5000), MW 20kDa (M-SH-20K), MW 30kDa (M-SH-30K), and MW 40kDa (M-SH-40K), in 1g and 10g packing sizes.
Different MW of methoxy thiol PEG products may be available by custom synthesis, please email us at tech@jenkemusa.com for details on custom PEGs. JenKem Technology provides repackaging services for an additional fee, please contact us if you require a different package size than our catalog selection.
Bulk PEGs and GMP PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Wu, N., et al., Improved tribological performance of Al2O3 fiber at microscale via a WS2-PEG/WPU self-lubricating sizing agent, Applied Surface Science, V. 657, 2024.
- Shahbaz, S., et al., PEGylated mesoporous silica core–shell redox-responsive nanoparticles for delivering paclitaxel to breast cancer cells, International Journal of Pharmaceutics, V. 655, 2024.
- Robert, J., et al., Coiled-coil peptide-based assembly of a plasmonic core-satellite polymer-metal nanocomposite as an efficient photothermal agent for drug delivery applications, Journal of Colloid and Interface Science, 641, 2023.
- Robert, J, et al., Coiled-Coil Peptide-Based Assembly of a Plasmonic Core-Satellite Polymer-Metal Nanocomposite as an Efficient Photothermal Agent, NANOTODAY-D-22-00764, 2022.
- Zhang, Y., et al., DNA-assembled visible nanodandelions with explosive hydrogen-bond breakage achieving uniform intra-tumor distribution (UITD)-guided photothermal therapy, Biomaterials, 2022, 121381.
- Morales-Zavala, F., et al., In vivo micro computed tomography detection and decrease in amyloid load by using multifunctionalized gold nanorods: a neurotheranostic platform for Alzheimer’s disease. Biomaterials Science. 2021.
- Cai, X., et al., Synthesis of Au@ MOF Core-Shell Hybrids for Enhanced Photodynamic/Photothermal Therapy. Journal of Materials Chemistry B. 2021.
- Ahmad, T., et al. Development of wound healing scaffolds with precisely-triggered sequential release of therapeutic nanoparticles. Biomaterials science. 2021; 9(12):4278-88.
- Wang, X.-M., et al., Exposure-time-dependent subcellular staging of gold nanoparticles deposition and vesicle destruction in mice livers, Nanomedicine: Nanotechnology, Biology and Medicine, 2021, V. 34.
- Guo, T., et al., Highly-selective detection of EGFR mutation gene in lung cancer based on surface enhanced Raman spectroscopy and asymmetric PCR, Journal of Pharmaceutical and Biomedical Analysis, 2020, 190,113522.
- Liu, Y., et al., Cathodic protected Mn2+ by NaxWO3 nanorods for stable magnetic resonance imaging-guided tumor photothermal therapy, Biomaterials, 2020, V. 234.
- Rong, L., et al., Iron chelated melanin-like nanoparticles for tumor-associated macrophage repolarization and cancer therapy, Biomaterials, 2019.
- Adibnia, V., et al., Chitosan hydrogel micro-bio-devices with complex capillary patterns via reactive-diffusive self-assembly, Acta Biomaterialia, 2019.
- Zhang, J., et al., Real-time and in-situ monitoring of Abrin induced cell apoptosis by using SERS spectroscopy, Talanta, 2019, V. 195, P. 8-16.
- Li, X., et al., Efficient biofunctionalization of MoS2 nanosheets with peptides as intracellular fluorescent biosensor for sensitive detection of caspase-3 activity, Journal of Colloid and Interface Science, 2019.
- Han, L., et al., Surface-enhanced resonance Raman scattering guided brain tumor surgery showing prognostic benefit in rat models, ACS applied materials & interfaces, 2019.
- Xu, W., et al., PEGylated hydrazided gold nanorods for pH-triggered chemo/ photodynamic/ photothermal triple therapy of breast cancer, Acta Biomaterialia, 2018, V. 82, P. 171-183.
- DiTullio, B.T., et al., Surface modification of polyaniline nanorods with thiol-terminated poly (ethylene oxide), Colloid and Polymer Science, 2018, 296(4), pp.637-645.
- Luan, J., et al., Environmental Stability of Plasmonic Biosensors based on Natural vs. Artificial Antibody, Analytical chemistry, 2018.
- Shao, B., et al., Nanogapped Au (core)@ Au-Ag (shell) structures coupled with Fe3O4 Magnetic nanoparticles for the detection of Ochratoxin A., Analytica Chimica Acta, 2018.
- Wang, Y., et al., Enzymatically driven formation of palindromic DNA-Au nanoparticles for snowball assembly and colorimetric biosensing, Sensors and Actuators B: Chemical, 2018, 267, pp.328-335.
- Su, B., et al., Effect of Retro‐Inverso Isomer of Bradykinin on Size‐Dependent Penetration of Blood–Brain Tumor Barrier, Small, 2018.
- Peng, G., at al., N-terminal site-specific PEGylation enhances the circulation half-life of Thymosin alpha 1, Journal of Drug Delivery Science and Technology, 2018.
- Li, H., et al., Combination of active targeting, enzyme-triggered release and fluorescent dye into gold nanoclusters for endomicroscopy-guided photothermal/photodynamic therapy to pancreatic ductal adenocarcinoma, Biomaterials, 2017.
- Liu, D., et al., A Fully Integrated Distance Readout ELISA-Chip for Point-of-Care Testing with Sample-in-Answer-out Capability, Biosensors and Bioelectronics, 2017.
- Yue, Q., et al., An EGFRvIII targeted dual-modal gold nanoprobe for imaging-guided brain tumor surgery, Nanoscale, 2017.
- Kampert, T., et al., Phenotypically Screened Carbon Nanoparticles for Enhanced Combinatorial Therapy in Triple Negative Breast Cancer, Cellular and Molecular Bioengineering, 2017, 1-6.
- Billingsley, M.M., et al., Antibody-nanoparticle conjugates to enhance the sensitivity of ELISA-based detection methods, PloS one, 2017, 12(5):e0177592.
- Li, S.S., et al., Revealing chemical processes and kinetics of drug action within single living cells via plasmonic Raman probes, Scientific Reports, 2017, 7.
- Srivastava, I., et al., Surface chemistry of carbon nanoparticles functionally select their uptake in various stages of cancer cells, Nano Research, 2017, 1-6.
- Ni, D., et al., Oxygen Vacancy Enables Markedly Enhanced Magnetic Resonance Imaging-Guided Photothermal Therapy of a Gd (3+)-Doped Contrast Agent, ACS Nano, 2017.
- Ma, N., et al., Shape-Dependent Radiosensitization Effect of Gold Nanostructures in Cancer Radiotherapy: Comparison of Gold Nanoparticles, Nanospikes, and Nanorods. ACS Applied Materials & Interfaces, 2017, 9(15):13037-48.
- Ho, L.W., Yet al., Effect of Alkylation on the Cellular Uptake of Polyethylene Glycol-Coated Gold Nanoparticles. ACS Nano, 2017.
- Chen, Q., et al., Surface-Enhanced Raman Scattering from Self-Assembled Film of Thiolated Peg-Modified Gold Nanoparticles, Journal of Applied Spectroscopy, 2017, 84(3):407-12.
- Garcia, J., New strategies based on nanosystems to facilitate the crossing through biological barriers, Universitat de Barcelona, 2016.
- Kennedy, S., et al., Sequential release of nanoparticle payloads from ultrasonically burstable capsules, Biomaterials, 2016, 75, P. 91-101.
- Liu, H., et al., Microfluidic synthesis of QD-encoded PEGDA microspheres for suspension assay, J. Mater. Chem. B, 2016.
- Wang, Y.L., et al., The ordering alignment of gold nanorods in liquid crystals and its applications to polarization-sensitive SERS. InJournal of Physics: Conference Series, 2016, 680:1, p. 012021.
- Zhang, B., et al., Conductive and protein resistant polypyrrole films for dexamethasone delivery, Journal of Materials Chemistry B, 2016.
- Shendi, D., et al., Tunable, Bioactive Protein Conjugated Hyaluronic Acid Hydrogels for Neural Engineering Applications. Journal of Materials Chemistry B., 2016.
- Zhang, Q., et al., Gold Nanoparticle (AuNP)-Based Surface-Enhanced Raman Scattering (SERS) Probe of Leukemic Lymphocytes, Plasmonics, 2016, 1-8.
- Tian, L., et al., Plasmonic Nanogels for Unclonable Optical Tagging, ACS Applied Materials & Interfaces, 2016, 8 (6), 4031-4041.
- Hu, W., et al., Redox and pH dual responsive poly(amidoamine) dendrimer-poly(ethylene glycol) conjugates for intracellular delivery of doxorubicin, Acta Biomaterialia, 2016, 36, p: 241-253.
- Ding, H., et al., Intracellular Temperature Imaging in Gold Nanorod-Assisted Photothermal Therapy with Luminescent Eu (III) Chelate Nanoparticles. Journal of Nanoscience and Nanotechnology, 2016, 16(4):3877-82.
- Wang, F., et al., Polydopamine‐Functionalized Graphene Oxide Loaded with Gold Nanostars and Doxorubicin for Combined Photothermal and Chemotherapy of Metastatic Breast Cancer, Advanced Healthcare Materials, 2016.
- Li, J., et al., SERS-active Plasmonic Nanoparticles with Ultra-small Interior Nanogap for Multiplex Quantitative Detection and Cancer Cell Imaging, Analytical Chemistry, 2016.
- Pickenhahn, V. D., Regioselective thioacetylation of chitosan end-groups for nanoparticle gene delivery systemset al., Chem. Sci., 2015, 6, 4650-4664.
- Fu, H., et al., Tumor‐Targeted Paclitaxel Delivery and Enhanced Penetration Using TAT ‐Decorated Liposomes Comprising Redox‐Responsive Poly(Ethylene Glycol), Journal of Pharmaceutical Science, 2015, 104(3): 1160-1173.
- Sun, Y., et al., Patterning of graphite nanocones for broadband solar spectrum absorption, AIP Advances, 2015, 5, 067139.
- Yuan, Y., et al., Topological nanocolloids with facile electric switching of plasmonic properties, Opt. Lett., 2015, 40, 5630-5633.
- Wang, S., et al., Three-Photon Luminescence of Gold Nanorods and Its Applications for High Contrast Tissue and Deep In Vivo Brain Imaging. Theranostics, 2015, 5(3), 251-266.
- Li, J., et al., Simple and Rapid Functionalization of Gold Nanorods with Oligonucleotides Using an mPEG-SH/Tween 20-Assisted Approach, Langmuir, 2015, 31 (28), 7869-7876.
- Zhang, B., et al., Functionalised inherently conducting polymers as low biofouling materials, Biofouling, 2015, 31:6.
- Wang, L., et al., Sea-Urchin-Like Au Nanocluster with Surface-Enhanced Raman Scattering in Detecting Epidermal Growth Factor Receptor (EGFR) Mutation Status of Malignant Pleural Effusion, ACS Applied Materials & Interfaces, 2015, 7 (1), 359-369.
- Gao, L., et al., Plasmon-Mediated Generation of Reactive Oxygen Species from Near-Infrared Light Excited Gold Nanocages for Photodynamic Therapy in Vitro ACS Nano, 2014, 8(7), p: 7260–7271.
- Li, J., Synergetic Approach for Simple and Rapid Conjugation of Gold Nanoparticles with Oligonucleotides, ACS Appl. Mater. Interfaces, 2014, 6(19), p. 16800–16807.
- Mei, L., et al., Increased tumor targeted delivery using a multistage liposome system functionalized with RGD, TAT and cleavable PEG. International Journal of Pharmaceutics, 2014, 468(1–2), p. 26-38.
- Hu, W., et al., Redox and pH-responsive poly (amidoamine) dendrimer-poly (ethylene glycol) conjugates with disulfide linkages for efficient intracellular drug release, Colloids Surf B Biointerfaces, 2014, 123, p:254-63.
- Ai, X., et al., Thin-film hydration preparation method and stability test of DOX-loaded disulfide-linked polyethylene glycol 5000-lysine-di-tocopherol succinate nanomicelles, Asian Journal of Pharmaceutical Sciences, 2014, 9(5), p: 244-250.
- Cui, J., et al., Nanoscale engineering of low-fouling surfaces through polydopamine immobilisation of zwitterionic peptides, Soft Matter, 2014, 10(15), 2656-2663.
- Chen, S., et al., Nonionic fluorosurfactant as an ideal candidate for one-step modification of gold nanorods, Nanoscale, 2014, 6, 3197-3205.
- Yuan, W., et al., Increased Delivery of Doxorubicin Into Tumor Cells Using Extracellularly Activated TAT Functionalized Liposomes: In Vitro and In Vivo Study, Journal of Biomedical Nanotechnology, 2014, 10:8, p. 1563-1573(11).
- Tang, J., et al., Liposomes co-modified with cholesterol anchored cleavable PEG and octaarginines for tumor targeted drug delivery, Journal of Drug Targeting, 2014, 22:4.
- Ai, X., et al., Star-Shape Redox-Responsive PEG-Sheddable Copolymer of Disulfide-Linked Polyethylene Glycol-Lysine-di-Tocopherol Succinate for Tumor-Triggering Intracellular Doxorubicin Rapid Release: Head-to-Head Comparison, Macromol. Biosci., 2014, 14: 1415–1428.
- Ni, D., et al., Dual-targeting upconversion nanoprobes across the blood–brain barrier for magnetic resonance/fluorescence imaging of intracranial glioblastoma, ACS nano, 2014, 8(2):1231-42.
- Yang, L., et al., Luminescent Ru(bpy)3 2+-doped silica nanoparticles for imaging of intracellular temperature, Microchimica Acta, 2013, 181:7, p. 743-749.
- Taylor, W., et al., Protein Adsorption on Well-Characterized Polyethylene Oxide Brushes on Gold: Dependence on Molecular Weight and Grafting Density, Langmuir, 2013, 29(20), p: 6116–6122.
- Molino, P. J., et al., Surface modification of polypyrrole/biopolymer composites for controlled protein and cellular adhesion, Biofouling, 2013, 29 (10), 1155-1167.
- Yue, Z., et al., PEGylation of platinum bio-electrodes. Electrochemistry Communications, 2013, 27, 54-58.
- Qin, H., et al., Gadolinium (III)-gold nanorods for MRI and photoacoustic imaging dual-modality detection of macrophages in atherosclerotic inflammation, Nanomedicine, 2013, 8.10: 1611-1624.
- Kuai, R., et al., Targeted Delivery of Cargoes into a Murine Solid Tumor by a Cell-Penetrating Peptide and Cleavable Poly(ethylene glycol) Comodified Liposomal Delivery System via Systemic Administration, Mol. Pharmaceutics, 2011, 8(6), p: 2151–2161.
- Wang, B., et al., Polymer Single Crystal Templated Janus Nanoparticles, Macromol. Rapid Commun., 2010, 31: 169–175.
- Tsai, D.H., et al., Competitive Adsorption of Thiolated Polyethylene Glycol and Mercaptopropionic Acid on Gold Nanoparticles Measured by Physical Characterization Methods, Langmuir, 2010, 26 (12), 10325-10333.
- Kuai, R., et al., Efficient Delivery of Payload into Tumor Cells in a Controlled Manner by TAT and Thiolytic Cleavable PEG Co-Modified Liposomes, Molecular Pharmaceutics, 2010, 7 (5), 1816-1826.
- Taylor, W., et al., Producing High-Density High-Molecular-Weight Polymer Brushes by a “Grafting to” Method from a Concentrated Homopolymer Solution, Langmuir, 2010, 26 (17), 13954-13958.
- Li, B., et al., Programmable Nanoparticle Assembly via Polymer Single Crystals, Macromolecules, 2009, 42 (24), 9394-9399.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom polymer synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7 guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.