 JenKem Technology provides high quality activated multi-arm polyethylene glycol derivatives (PEGs) for hydrogels, with high purity and low polydispersity.
JenKem Technology provides high quality activated multi-arm polyethylene glycol derivatives (PEGs) for hydrogels, with high purity and low polydispersity.
JenKem Technology’s multi-arm PEG derivatives can be cross-linked into hydrogels. PEG hydrogels have a variety of applications in medical devices and regenerative medicine, and are especially of interest for controlled release of drugs, for 3D cell culture, and for wound sealing and healing [1].
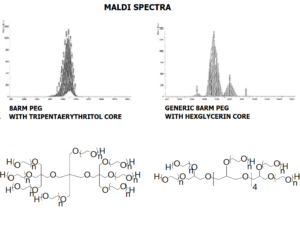
JenKem Technology’s multi-arm star PEGs are synthesized by ethoxylation of tripentaerythritol (8ARM(TP) PEG), hexaglycerol (8ARM PEG), dipentaerythritol (6ARM PEG), pentaerythritol (4ARM PEG), or glycerol (3ARM PEG). The number of ethylene oxide units in the PEG chain may not be equal for all arms. The total molecular weight reported for the JenKem multi-arm PEGs is the sum of the molecular weights of the PEG chains on each arm. 8ARM(TP)-PEGs with tripentaerythritol core have a higher purity as evidenced by MALDI compared to the generic 8ARM-PEGs with a hexaglycerin core.
Multi-arm star PEG products with molecular weights and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
JenKem Technology provides GMP grade PEG derivatives and bulk orders via custom synthesis, offering the opportunity to match customers’ special quality requirements. JenKem Technology is capable of development and synthesis of a wide range of GMP PEG derivatives starting at 200g up to 40 kg or greater batches, under ISO 9001 and ISO 13485 certified quality management system, following ICH Q7A guidelines. For inquiries on cGMP production of PEG derivatives please contact us at tech@jenkemusa.com.
For global distribution, please visit link. Please click the buttons below to order directly from JenKem Technology:
3ARM PEG DERIVATIVES
| 3ARM PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | 3arm PEG Amine. Hydrogel PEG. Amine group binds to carboxylic group (-COOH) or other amine reactive chemical groups |
4ARM PEG DERIVATIVES
| 4ARM PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | 4arm PEG Amine, Free Amine. Hydrogel PEG. Amine group binds to carboxylic group (-COOH) or other amine reactive chemical groups [2, 6, 8] | |
| ≥ 95% | 4arm PEG Amine, HCl Salt. Hydrogel PEG. Amine group binds to carboxylic group (-COOH) or other amine reactive chemical groups [3-5] | |
| ≥ 95% | 4arm PEG Carboxyl (4arm PEG Acetic Acid, 4arm-COOH, 4arm-CM). Hydrogel PEG. Carboxyl group binds to amino or other acid reactive chemical groups [7] | |
| ≥ 95% | 4arm PEG SCM (4arm PEG NHS Ester). Hydrogel PEG. This is the activated form of 4ARM-COOH. [8, 9] | |
| ≥ 95% | 4arm PEG Succinimidyl Glutaramide. Hydrogel PEG. SGA has a longer hydrolysis half-life compared with SCM [23]. | |
| ≥ 95% | 4arm PEG Nitrophenyl Carbonate. Hydrogel PEG. Carbonate linker between PEG and NHS ester; the reaction with amine groups releases p-nitrophenol which can be easily traced by UV spectroscopy. | |
| > 90% | 4arm PEG Succinimidyl Carbonate. Hydrogel PEG. Carbonate linker between PEG and NHS ester; longer hydrolysis half-life compared with SCM | |
| > 90% | 4arm PEG Maleimide. Hydrogel PEG. Maleimide is selective for thiol groups and reacts at pH 5.0-6.5. [24-27] | |
| ≥ 95% | 4arm PEG Acrylate. Hydrogel PEG. Used in vinyl polymerization or co-polymerization [10, 47] | |
| > 90% | 4arm PEG Thiol. Hydrogel PEG. Selective for thiol groups under mild reaction conditions [11] | |
| > 90% | 4arm PEG Vinylsulfone. Hydrogel PEG. VS binds free thiol groups in aqueous buffer between pH 6.5~8.5 at room temperature [12] | |
| ≥ 95% | 4arm PEG Succinimidyl Succinate. Hydrogel PEG. Cleavable PEG linker. The ester linker between PEG and NHS ester enables the feature of “degradable hydrogel”. [28] | |
| ≥ 95% | 4arm PEG Succinimidyl Glutarate. Hydrogel PEG. Cleavable PEG linker. The ester linker between PEG and NHS ester enables the feature of “degradable hydrogel”. [13]. | |
| > 90% | 4arm PEG Isocianate. Hydrogel PEG. NCO group is useful for coupling hydroxyl through a stable urethane linker | |
| ≥ 95% | 4arm PEG Azide. Hydrogel PEG. Azide group reacts with alkynes in aqueous solution catalyzed by copper [14] |
4ARM PEG RAW MATERIALS
| 4ARM PEG RAW MATERIALS | MAIN PEAK FRACTION BY GPC | POLYDISPERSITY BY GPC |
|---|---|---|
| ≥ 95% | ≤ 1.05 |
6ARM PEG DERIVATIVES
| 6ARM PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | 6arm PEG Amine. Crosslinks into hydrogels. Amine group reacts with carboxylic group (-COOH) or other amine reactive chemical groups [15] |
6ARM PEG RAW MATERIALS
| 6ARM PEG RAW MATERIALS | MAIN PEAK FRACTION BY GPC | POLYDISPERSITY BY GPC |
|---|---|---|
| ≥ 95% | ≤ 1.08 |
8ARM PEG DERIVATIVES WITH TRIPENTAERYTHRITOL CORE
| 8ARM PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | 8arm PEG Amine. Crosslinks into hydrogels. Amine group reacts with carboxylic group (-COOH) or other amine reactive chemical groups . 8ARM(TP)-PEG with tripentaerythritol core has a lower polydispersity and higher molecular weight accuracy compared with the generic 8ARM-PEG with a hexaglycerol core.[16] | |
| ≥ 95% | 8arm PEG Carboxyl. Crosslinks into hydrogels. Carboxyl group reacts with amino or other acid reactive chemical groups. 8ARM(TP)-PEG with tripentaerythritol core has a lower polydispersity and higher molecular weight accuracy compared with the generic 8ARM-PEG with a hexaglycerol core. [29-31] | |
| > 90% | 8arm PEG Maleimide. Crosslinks into hydrogels. MAL is selective for thiol groups on cystein side chains; reacts at pH 5.0-6.5. 8ARM(TP)-PEG with tripentaerythritol core has a lower polydispersity and higher molecular weight accuracy compared with the generic 8ARM-PEG with a hexaglycerin core. [32] | |
| ≥ 95% | 8arm PEG Acrylate. Crosslinks into hydrogels. Used in vinyl polymerization or co-polymerization. 8ARM(TP)-PEG with tripentaerythritol core has a lower polydispersity and higher molecular weight accuracy compared with the generic 8ARM-PEG with a hexaglycerol core. [33] | |
| > 90% | 8arm PEG Thiol. Crosslinks into hydrogels. Selective for thiol groups under mild reaction conditions. 8ARM(TP)-PEG with tripentaerythritol core has a lower polydispersity and higher molecular weight accuracy compared with the generic 8ARM-PEG with a hexaglycerol core. [34, 35] | |
| > 90% | 8arm PEG Vinylsulfone. Crosslinks into hydrogels. VS reacts with free thiol groups in aqueous buffer between pH 6.5~8.5 at room temperature. 8ARM(TP)-PEG with tripentaerythritol core has a lower polydispersity and higher molecular weight accuracy compared with the generic 8ARM-PEG with a hexaglycerol core. [36-38] | |
| ≥ 95% | 8arm PEG Succinimidyl Succinate. Crosslinks into hydrogels. Cleavable PEG linker. The ester linker between PEG and NHS ester facilitates the formation of degradable hydrogel. 8ARM(TP)-PEG with tripentaerythritol core has a lower polydispersity and higher molecular weight accuracy compared with the generic 8ARM-PEG with a hexaglycerol core. [39] | |
| ≥ 95% | 8arm PEG Succinimidyl Glutarate. Crosslinks into hydrogels. Cleavable PEG linker. The ester linker between PEG and NHS ester facilitates the formation of degradable hydrogel. 8ARM(TP)-PEG with tripentaerythritol core has a lower polydispersity and higher molecular weight accuracy compared with the generic 8ARM-PEG with a hexaglycerol core. [40] | |
| > 85% | 8arm PEG Norbornene. Crosslinks into hydrogels. Norbornene NB PEGs are suitable for copper-free click chemistry reactions with tetrazines and for thiol-ene click reactions with thiols. 8ARM(TP)-PEG with tripentaerythritol core has a lower polydispersity and higher molecular weight accuracy compared with the generic 8ARM-PEG with a hexaglycerol core. [41-44] | |
8ARM PEG DERIVATIVES WITH HEXAGLYCEROL CORE
| 8ARM PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | 8arm PEG Amine. Crosslinks into hydrogels. Amine group reacts with carboxylic group (-COOH) or other amine reactive chemical groups [17-18] | |
| ≥ 95% | 8arm PEG Carboxyl. Crosslinks into hydrogels. Carboxyl group binds amino or other acid reactive chemical groups [16] | |
| > 90% | 8arm PEG Maleimide. Crosslinks into hydrogels. MAL group is selective for thiol groups; reacts at pH 5.0-6.5.[19, 46]. | |
| ≥ 95% | 8arm PEG Acrylate. Crosslinks into hydrogels. Used in vinyl polymerization or co-polymerization [20] | |
| > 90% | 8arm PEG Thiol. Crosslinks into hydrogels. Selective for thiol groups under mild reaction conditions [21] | |
| ≥ 95% | 8arm PEG Succinimidyl Succinate. Crosslinks into hydrogels. Cleavable PEG linker. The ester linker between PEG and NHS ester facilitates the formation of degradable hydrogel. Binds to amino group of lysine(s) or N-terminal amines [39, 45] | |
| ≥ 95% | 8arm PEG Succinimidyl Glutarate. Crosslinks into hydrogels. Cleavable PEG linker. The ester linker between PEG and NHS ester facilitates the formation of degradable hydrogel [22] |
8ARM PEG RAW MATERIALS
| 8ARM PEG RAW MATERIALS | MAIN PEAK FRACTION BY GPC | POLYDISPERSITY BY GPC |
|---|---|---|
| ≥ 95%(> 90% for MW 40000 Da TP core) | ≤ 1.08 (TP core) (≤ 1.12 (hexaglycerol core)) |
For information on multi-arm heterobifunctional PEGs please visit:
MULTIARM HETEROBIFUNCTIONAL PEGs
References:
- Hutanu, D., et al., Recent Applications of Polyethylene Glycols (PEGs) and PEG Derivatives. Mod Chem appl, 2014, 2(132).
- Baker, A., et al., Stable oxime-crosslinked hyaluronan-based hydrogel as a biomimetic vitreous substitute, Biomaterials, 2021, V. 271.
- Lee, Y.Y., et al, Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm, Biomaterials, 2021, 267, 120389.
- Ding, Y. et al, Tethering transforming growth factor β1 to soft hydrogels guides vascular smooth muscle commitment from human mesenchymal stem cells, Acta Biomaterialia, 2020, V. 105, P. 68-77.
- Newland, B., et al., Static and dynamic 3D culture of neural precursor cells on macroporous cryogel microcarriers, MethodsX, 2020, V.7.
- Bachmann, D., et al., Retargeting of UniCAR T cells with an in vivo synthesized target module directed against CD19 positive tumor cells, Oncotarget, 2018, 9(7), p.7487.
- Dai, L., et al., Self-assembled targeted folate-conjugated eight-arm-polyethylene glycol–betulinic acid nanoparticles for co-delivery of anticancer drugs, J. Mater. Chem. B, 2015, 3, 3754-3766.
- Mou, C., et al., Electrochemical-mediated gelation of catechol-bearing hydrogels based on multimodal crosslinking, Journal of Materials Chemistry B., 2019.
- Ding, Y., et al., Biomimetic soft fibrous hydrogels for contractile and pharmacologically responsive smooth muscle, Acta biomaterialia, 2018.
- Huang, Y., et al., Structural aspects controlling the mechanical and biological properties of tough, double network hydrogels. Acta Biomaterialia. 2022.
- Li, J., et al., Network-Based Redox Communication Between Abiotic Interactive Materials, iScience, 2022.
- Kumar, M., et al., A fully defined matrix to support a pluripotent stem cell derived multi-cell-liver steatohepatitis and fibrosis model, Biomaterials, 2021, 121006.
- Pereira, D.R., et al., Macromolecular modulation of a 3D hydrogel construct differentially regulates human stem cell tissue-to-tissue interface, Biomaterials Advances, 2022, 133.
- DeForest, C.A., E.A. Sims, and K.S. Anseth, Peptide-Functionalized Click Hydrogels with Independently Tunable Mechanics and Chemical Functionality for 3D Cell Culture. Chemistry of Materials, 2010, 22(16): p. 4783-4790.
- Chong, Y., et al., The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials, 2014, 35(19): p. 5041-5048.
- Wang, M., et al., A surface convertible nanoplatform with enhanced mitochondrial targeting for tumor photothermal therapy, Colloids and Surfaces B: Biointerfaces, 2020, V.189.
- Chauhan, N., et al., Dexamethasone-loaded, injectable pullulan-poly(ethylene glycol) hydrogels for bone tissue regeneration in chronic inflammatory conditions, Materials Science and Engineering: C, 2021, V. 130
- Schroeder, M. E., et al., Osteopontin activity modulates sex‐specific calcification in engineered valve tissue mimics, Bioengineering & translational medicine 2023, 8.1, e10358.
- Stock, A.A., et al., Conformal Coating of Stem Cell-Derived Islets for β Cell Replacement in Type 1 Diabetes, Stem Cell Reports, 2020, 14(1), P. 91-104
- Carleton, M. M., et al., Methacrylic acid-based hydrogels enhance skeletal muscle regeneration after volumetric muscle loss in mice, Biomaterials, 2021, V. 275.
- Swaminathan, G., et al., Effect of substrate stiffness on human intestinal enteroids’ infectivity by enteroaggregative Escherichia coli, Acta Biomaterialia, 2021, V. 132.
- Kaphle, P., et al., The mechanical and pharmacological regulation of glioblastoma cell migration in 3D matrices, Journal of cellular physiology, 2019, 234(4):3948-60.
- Buwalda, S.J., et al., Ultrafast in situ forming poly (ethylene glycol)-poly (amido amine) hydrogels with tunable drug release properties via controllable degradation rates, European Journal of Pharmaceutics and Biopharmaceutics, 2019, 139:232-9.
- Schirmer, L., et al., Glycosaminoglycan-based hydrogels with programmable host reactions, Biomaterials, 2020, V. 228.
- Scott, R. A., et al., Substrate stiffness directs the phenotype and polarization state of cord blood derived macrophages, Acta Biomaterialia, 2021, V. 122, P. 220-235.
- Bock N, et al., GelMA, Click-Chemistry Gelatin and Bioprinted Polyethylene Glycol-Based Hydrogels as 3D Ex Vivo Drug Testing Platforms for Patient-Derived Breast Cancer Organoids. Pharmaceutics. 2023; 15(1):261.
- Li, H., et al., Synthesis of thiol-terminated PEG-functionalized POSS cross-linkers and fabrication of high-strength and hydrolytic degradable hybrid hydrogels in aqueous phase, European Polymer Journal, 2019, 116:74-83.
- Rane, A.A., Understanding mechanisms by which injectable biomaterials affect cardiac function postmyocardial infarction, UC San Diego, 2012.
- Maisonneuve, B. G. C., et al., Effects of Synthetic Biomacromolecule Addition on the Flow Behavior of Concentrated Mesenchymal Cell Suspensions, Biomacromolecules 2015, 16(1): 275-283.
- Dai, L., et al., Self-assembled targeted folate-conjugated eight-arm-polyethylene glycol–betulinic acid nanoparticles for co-delivery of anticancer drugs, J. Mater. Chem. B, 2015, 3, 3754-3766.
- Liu, Ke-Feng, et al., Design, synthesis and in vivo antitumor efficacy of novel eight-arm-polyethylene glycol–pterostilbene prodrugs, RSC Adv., 2015, 5, 51592-51599.
- Shatz, W., et al., Identification and characterization of an octameric PEG-protein conjugate system for intravitreal long-acting delivery to the back of the eye. PLoS One, 2019, 14(6), p.e0218613.
- Wei, Z., et al., 3D Printing of PEG Hydrogel Scaffolds Using Novel Low Toxicity Photoinitiator, 2018, Society for Biomaterials Meeting poster presentation.
- Rao, V.V., et al., Rescuing mesenchymal stem cell regenerative properties on hydrogel substrates post serial expansion, Bioengineering & translational medicine, 2019.
- Nguyen, M.K., et al., RNA interfering molecule delivery from in situ forming biodegradable hydrogels for enhancement of bone formation in rat calvarial bone defects, Acta Biomaterialia, V. 75, 2018, P. 105-114.
- Tomaszewski, C. E., et al., Sequestered cell-secreted extracellular matrix proteins improve murine folliculogenesis and oocyte maturation for fertility preservation, Acta Biomaterialia, 2021.
- Kinney, S.M., et al., Degradable methacrylic acid-based synthetic hydrogel for subcutaneous islet transplantation, Biomaterials, 2022, V. 281.
- Yu, W., et al., Light‐Addressable Nanocomposite Hydrogels Allow Plasmonic Actuation and In Situ Temperature Monitoring in 3D Cell Matrices. Advanced Functional Materials. 2021, 2108234.
- Giorgi, M.E., et al., Improved bioavailability of inhibitors of Trypanosoma cruzi trans-sialidase: PEGylation of lactose analogs with multiarm polyethyleneglycol. Glycobiology, 2012, 22(10): p. 1363-1373.
- Na, K.-S., et al., Effect of mesenchymal stromal cells encapsulated within polyethylene glycol-collagen hydrogels formed in situ on alkali-burned corneas in an ex vivo organ culture model, Cytotherapy, 2021, V. 23 (6), P. 500-509.
- Kim MH, et al., Poly (ethylene glycol)–Norbornene as a Photoclick Bioink for Digital Light Processing 3D Bioprinting. ACS Applied Materials & Interfaces. 2023.
- Luo, Y., et al., Light-induced dynamic RGD pattern for sequential modulation of macrophage phenotypes, Bioactive Materials, 2021, V. 6(11), P. 4065-4072.
- Ortiz-Cárdenas, J.E., et al., Towards spatially-organized organs-on-chip: Photopatterning cell-laden thiol-ene and methacryloyl hydrogels in a microfluidic device. Organs-on-a-Chip, 2022, 100018.
- Khang, A., et al., Three-dimensional analysis of hydrogel-imbedded aortic valve interstitial cell shape and its relation to contractile behavior. Acta Biomaterialia, 2022.
- Ju, Y., et al., Engineered Metal-Phenolic Capsules Show Tunable Targeted Delivery to Cancer Cells. Biomacromolecules, 2016.
- Zhang H., et al., Reprogramming of Activated Pancreatic Stellate Cells via Mechanical Modulation of Transmembrane Force-sensitive N-cadherin Receptor. Journal of Molecular Biology. 2023; 435(1):167819.
- Sun, X., et al., Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus, Bioactive Materials, 6(3), 2021, P. 757-769.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.
