PEG products with additional MW may be made to order, please contact us for details
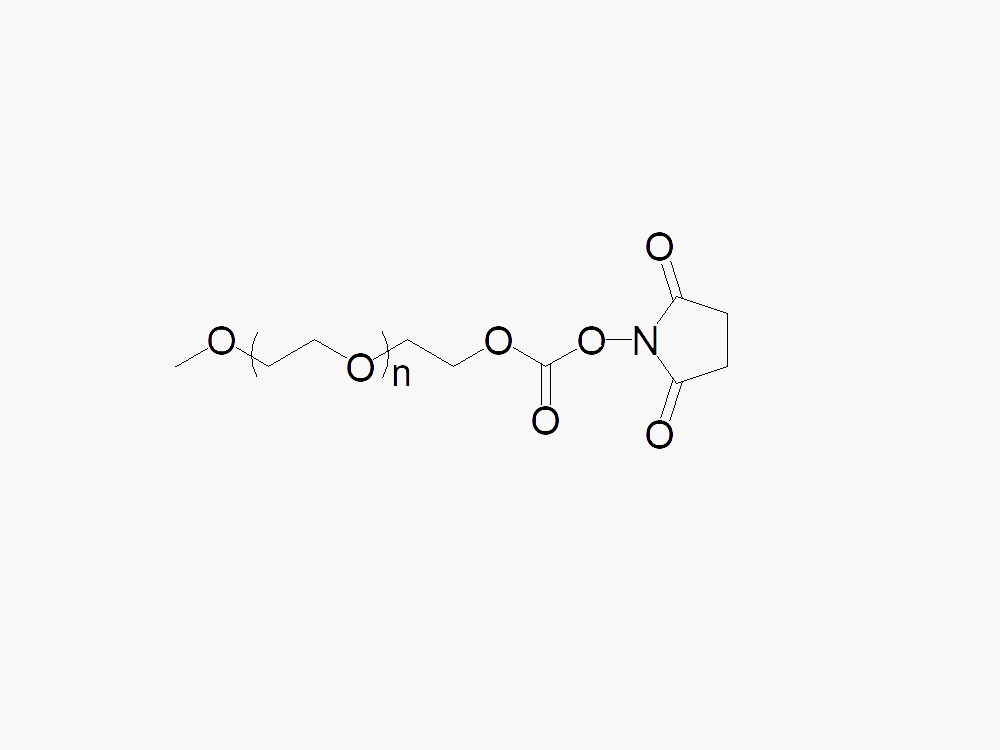
Methoxy PEG Succinimidyl Carbonate
$80.00 – $640.00
Description
Methoxy PEG Succinimidyl Carbonate for amine PEGylation, with superior quality specification of ≥95% Substitution.
JenKem Technology’s Methoxy PEG Succinimidyl Carbonate (Methoxy PEG SC) is a high quality amine reactive NHS PEG product which reacts with the amine group of lysine(s) such as the lysines present on IFN, Interferon alpha 2b, by means of stable urethane linkages. Methoxy PEG Succinimidyl Carbonate has a longer hydrolysis half-life compared with M-PEG-SCM. JenKem Technology offers Methoxy PEG Succinimidyl Carbonate with MW 10000 Da (M-SC-10K), MW 12000 (M-SC-12K), and MW 20000 Da (M-SC-20K), in 1g and 10g packing sizes.
Different MW of Methoxy PEG Succinimidyl Carbonate products may be available by custom synthesis, please email us at tech@jenkemusa.com for details on custom PEGs. Other related linear Amine Reactive PEG products are available – please select linear carbonate PEGs; cleavable linker NHS PEGs; or stable linker NHS PEGs. JenKem Technology provides repackaging services for an additional fee, please contact us if you require a different package size than our catalog selection.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Saad, M. A., et al., Dual-Function Antibody Conjugate-Enabled Photoimmunotherapy Complements Fluorescence and Photoacoustic Imaging of Head and Neck Cancer Spheroids, Bioconjugate Chemistry, V. 35 (1), 2024.
- Ren, M., et al., An oligopeptide/aptamer-conjugated dendrimer-based nanocarrier for dual-targeting delivery to bone. Journal of Materials Chemistry B. 2021, 9(12):2831-44.
- Luan, J., et al., GSDMD membrane pore is critical for IL-1β release and antagonizing IL-1β by hepatocyte-specific nanobiologics is a promising therapeutics for murine alcoholic steatohepatitis, Biomaterials, 2020, V. 227.
- Sousa, A., et al., Design of experiments to select triphenylphosphonium-polyplexes with suitable physicochemical properties for mitochondrial gene therapy, Journal of Molecular Liquids, 2020, V. 302.
- Yan, J., et al., Tumor Contrast Imaging with Gas Vesicles by Circumventing the Reticuloendothelial System, Ultrasound in Medicine & Biology, 2020, V. 46(2); P. 359-368.
- Vaillard, V.A., et al., mPEG–NHS carbonates: Effect of alkyl spacers on the reactivity: Kinetic and mechanistic insights, Journal of Applied Polymer Science, 2019, 136(5):47028.
- Ma, S.S., et al., The pharmacokinetic and pharmacodynamic properties of site-specific pegylated genetically modified recombinant human interleukin-11 in normal and thrombocytopenic monkeys, European Journal of Pharmaceutics and Biopharmaceutics, 2017.
- Zhao, Y.Z., et al., PEGylation with the thiosuccinimido butylamine linker significantly increases the stability of haloalkane dehalogenase DhaA, Journal of Biotechnology, 2017.
- Lee, K., et al., Enhanced accumulation of theranostic nanoparticles in brain tumor by external magnetic field mediated in situ clustering of magnetic nanoparticles, Journal of Industrial and Engineering Chemistry, 2017.
- Wan, X., et al., Effect of protein immunogenicity and PEG size and branching on the anti-PEG immune response to PEGylated proteins, Process Biochemistry, 2017, 52:183-91.
- Fahrlander E., et al., PEGylated human serum albumin (HSA) nanoparticles: preparation, characterization and quantification of the PEGylation extent, Nanotechnology, 2015, 26(14):145103.
- Zhang, J., et al., Magnetic Targeting of Novel Heparinized Iron Oxide Nanoparticles Evaluated in a 9L-glioma Mouse Model, Pharmaceutical Research, 2014, 31:3, pp 579-592.
- Peng, F., et al., PEGylation of G-CSF in organic solvent markedly increase the efficacy and reactivity through protein unfolding, hydrolysis inhibition and solvent effect. Journal of Biotechnology, 2014, 170: p. 42-49.
- Mingji Jin, et al, Preparation of pegylated lumbrokinase and an evaluation of its thrombolytic activity both in vitro and in vivo, Acta Pharmaceutica Sinica B, 2013, 3(2) p: 123-129.
- Zhang, J., et al., Long-Circulating Heparin-Functionalized Magnetic Nanoparticles for Potential Application as a Protein Drug Delivery Platform, Molecular Pharmaceutics, 2013, 10(10), 3892-3902.
- Jun Wang, et al., An oriented adsorption strategy for efficient solid phase PEGylation of recombinant staphylokinase by immobilized metal-ion affinity chromatography, Process Biochemistry, 2012, 47, 1, p: 106-112.
- Liu, S.. et al., Mono-PEGylation of ribonuclease A: High PEGylation efficiency by thiolation with small molecular weight reagent, Process Biochemistry, 2012, 47(9), p: 1364-1370.
- Peng, F., et al., PEGylation of Proteins in Organic Solution: A Case Study for Interferon beta-1b, Bioconjugate Chem., 2012, 23 (9), 1812-1820.
- Beibei, H., Design, Preparation and in vitro Bioactivity of Mono-PEGylated Recombinant Hirudin, Chin. J. Chem. Eng., 2007, 15(6) 775—780.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization directly from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.