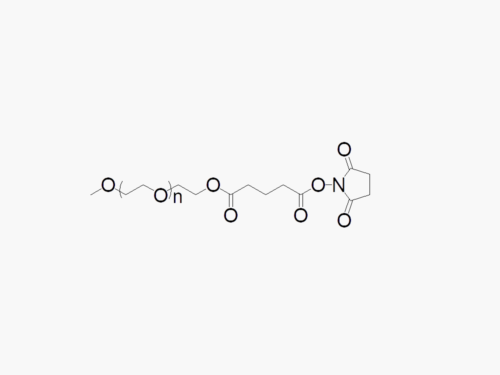
Please contact us for a quotation for large scale GMP manufacture.
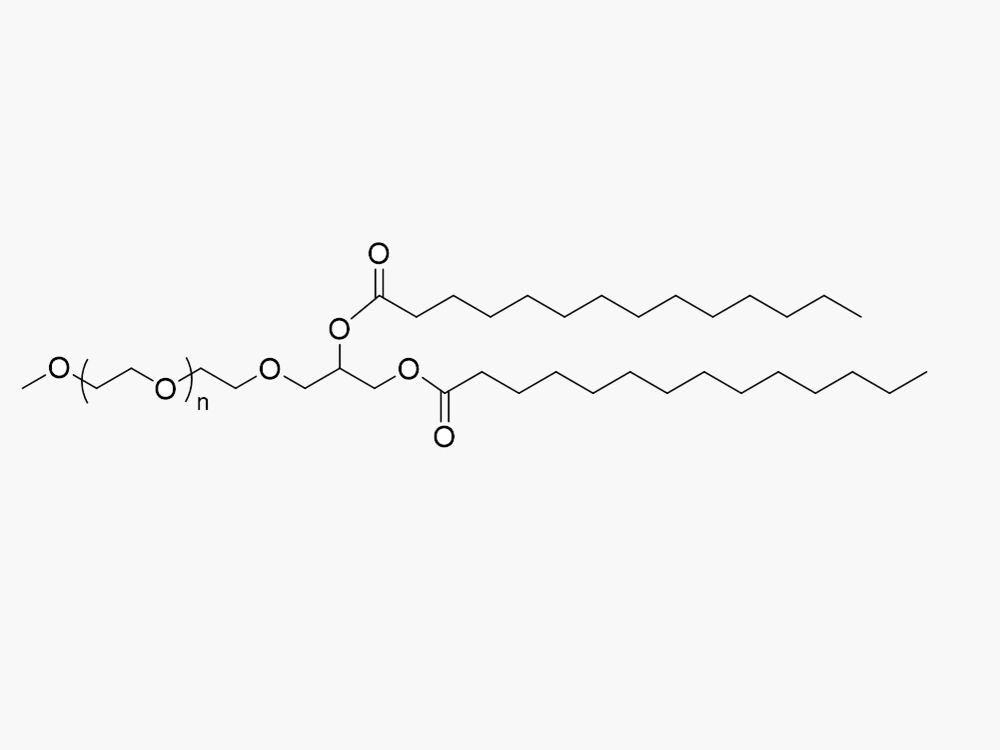
Methoxy PEG DMG
$100.00
Description
JenKem Technology provides Methoxy PEG DMG (MPEG DMG; Methoxy PEG Dimyristoyl-rac-glycero) products with high purity and substitution (≥ 95%), in both GMP and non-GMP grade, from small lab scale through large kilogram scale. MPEG DMG products are useful for lipid nanoparticles for mRNA delivery such as those used for Covid-19 vaccine manufacture1.
Lipid Nanoparticles (LNPs) are delivery systems commonly employed in the field of nucleic acid drugs. LNPs are generally believed to combine with the cell membrane through non-covalent affinity and absorbed by endocytosis. After being absorbed by the cell, the mRNA delivered by LNPs are released to the cytoplasm and express the target protein, escaping from the endocytosis. LNP technology has been adopted for COVID-19 vaccine manufacture1.
JenKem Technology has scaled up the GMP manufacture of mPEG DMG derivatives for LNPs, such as M-DMG-2000 (Methoxy PEG Dimyristoyl-rac-glycero, MW 2000; DMG-PEG-2000; CAS 1397695-86-1), and related PEG intermediates. Please contact us for a quotation.
To learn about other PEG products for RNA delivery, please visit this link.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7 guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.
References:
- Zhang, L., et al., Controlled production of liposomes with novel microfluidic membrane emulsification for application of entrapping hydrophilic and lipophilic drugs, Journal of Industrial and Engineering Chemistry, 131, 2024.
- Schoenmaker, Linde, et al., mRNA-lipid nanoparticle COVID-19 vaccine : structure and stability, International Journal of Pharmaceutics 601, 2021.