PEG products with additional MW may be made to order, please contact us for details
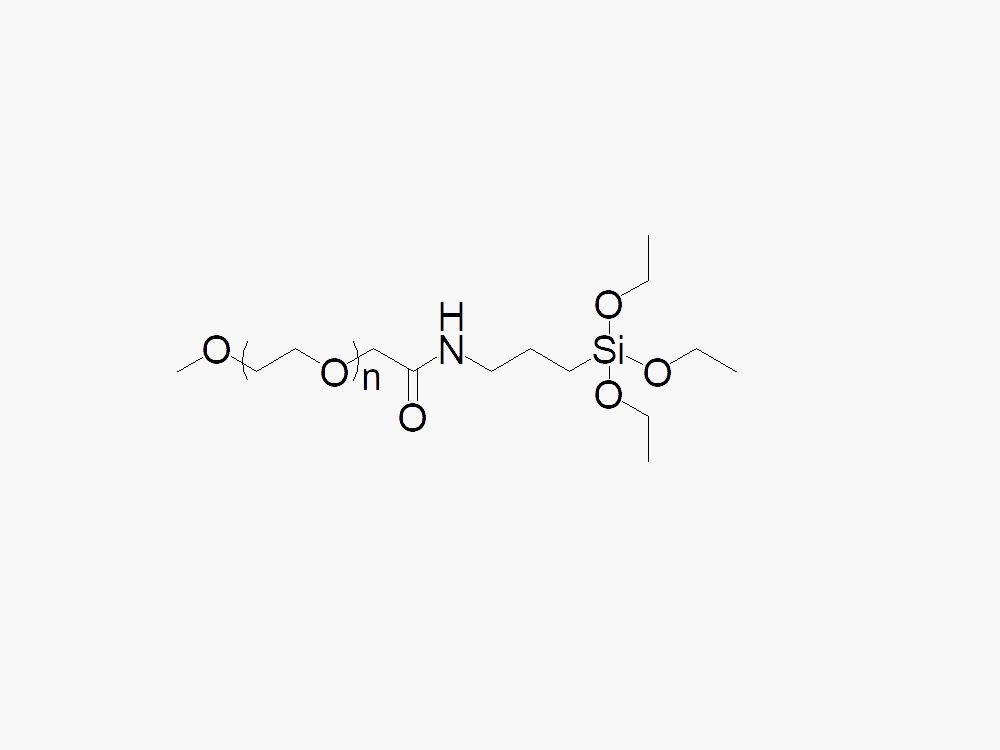
Methoxy PEG Silane
$130.00 – $1,040.00
Description
Methoxy PEG Silane with superior quality specification of ≥90% Substitution.
Methoxy PEG Silane from JenKem Technology is a high quality silane coupling PEG reagent (triethoxy silyl ether PEG) designed for glass / silica / metal surface modification and deactivation. Methoxy PEG Silane from JenKem Technology is available with MW 5,000 Da (M-SLN-5000) and MW 20,000 Da (M-SLN-20K), in 1g and 10g packing sizes. JenKem Technology provides repackaging services for an additional fee, please contact us if you require a different package size than our catalog selection.
Different MW of Methoxy PEG Silane products may be available by custom synthesis, please email us at tech@jenkemusa.com for details on custom PEGs. Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Chen, W., et al., Endoskeletal coacervates with mobile-immobile duality for long-term utility, Chemical Engineering Journal, V. 462, 2023.
- Li, C., et al., 3D-CEUS tracking of injectable chemo-sonodynamic therapy-enabled mop-up of residual renal cell carcinoma after thermal ablation, Materials Today Bio, V. 18, 2023.
- Skowyra, M. L., et al., Cell-free reconstitution of peroxisomal matrix protein import using Xenopus egg extract, STAR Protocols, V. 4(1), 2023.
- Sun, M, et al., Mesoporous silica nanoparticles inflame tumors to overcome anti-PD-1 resistance through TLR4-NFκB axis. Journal for ImmunoTherapy of Cancer. 2021, 9(6).
- Forman, MB, et al., Novel Guidewire Design and Coating for Continuous Delivery of Adenosine During Interventional Procedures. Journal of the American Heart Association. 2021, 10(3):e019275
- Nakashima, KK, Active coacervate droplets are protocells that grow and resist Ostwald ripening. Nature Communications. 2021, 12(1):1-1.
- Nakashima, K. K., et al., Chapter Thirteen – Enzymatic control over coacervation, Methods in Enzymology, Academic Press, 2021, 646, P. 353-389.
- Wang, S., et al.,. Endoplasmic Reticulum Network Formation with Xenopus Egg Extracts, Cold Spring Harbor Protocols, 2018.
- Wang, L, et al., Iron-engineered mesoporous silica nanocatalyst with biodegradable and catalytic framework for tumor-specific therapy, Biomaterials, 2018, V. 163, P. 1-13.
- Yu, L., et al., Ultrasmall mesoporous organosilica nanoparticles: Morphology modulations and redox-responsive biodegradability for tumor-specific drug delivery, Biomaterials, 2018, V. 161, P. 292-305.
- Field, C.M., et al., Chapter 24 – Xenopus extract approaches to studying microtubule organization and signaling in cytokinesis, In: Arnaud Echard, Editor(s), Methods in Cell Biology, Academic Press, 2017, V. 137, P. 395-435.
- Huang, P., et al., Molecularly organic/inorganic hybrid hollow mesoporous organosilica nanocapsules with tumor-specific biodegradability and enhanced chemotherapeutic functionality, Biomaterials, 2017, V. 125, P. 23-37.
- Zhou, M., One-pot synthesis of redox-triggered biodegradable hybrid nanocapsules with a disulfide-bridged silsesquioxane framework for promising drug delivery, Journal of Materials Chemistry B, 2017.
- Huang, P., et al., Metalloporphyrin-Encapsulated Biodegradable Nanosystems for Highly Efficient Magnetic Resonance Imaging-Guided Sonodynamic Cancer Therapy. Journal of the American Chemical Society, 2017, 139(3):1275-84.
- Mundoor, H., et al., Triclinic nematic colloidal crystals from competing elastic and electrostatic interactions, Science, 2016, 352, 69.
- Kobayashi, Y., et al., Preparation of Silica-Coated Quantum Dot Nanoparticle Colloid Solutions and Their Application in in-vivo Fluorescence Imaging, J. Chem. Eng. Japan, 2015, 48:2, p. 112-117.5.
- Mundoor, H., et al., Mesostructured Composite Materials with Electrically Tunable Upconverting Properties, Small, 2015, 11: 5572–5580.
- Field, CM, et al., Xenopus egg cytoplasm with intact actin, Methods in Enzymologie, 2014, 450.
- Kobayashi, Y., et al., Imaging Processes Using Core-Shell Particle Colloid Solutions for Medical Diagnosis, Athens Journal of Natural & Formal Sciences, 2014, 1(1) p.31-41.
- Schwarz, D. S., et al., The calcium-dependent ribonuclease XendoU promotes ER network formation through local RNA degradation, The Journal of cell biology, 2014, 207.1 : 41-57.
- Kobayashi, Y., et al., Preparation of AgI/Silica/Poly(Ethylene Glycol) Nanoparticle Colloid Solution and X-Ray Imaging Using It. ISRN Nanomaterials, 2013, p. 5.
- Scott, M.A., Ultra-rapid 2-D and 3-D laser microprinting of proteins. Diss. Massachusetts Institute of Technology, 2013.
- Kobayashi, Y., et al., In-vivo fluorescence imaging technique using colloid solution of multiple quantum dots/silica/poly(ethylene glycol) nanoparticles, Journal of Sol-Gel Science and Technology, 2013, 66:1, pp 31-37.
- Scott, M.A., Ultra-rapid laser protein micropatterning: screening for directed polarization of single neurons, Lab Chip, 2011.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom polymer synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.