PEG products with additional MW may be made to order, please contact us for details
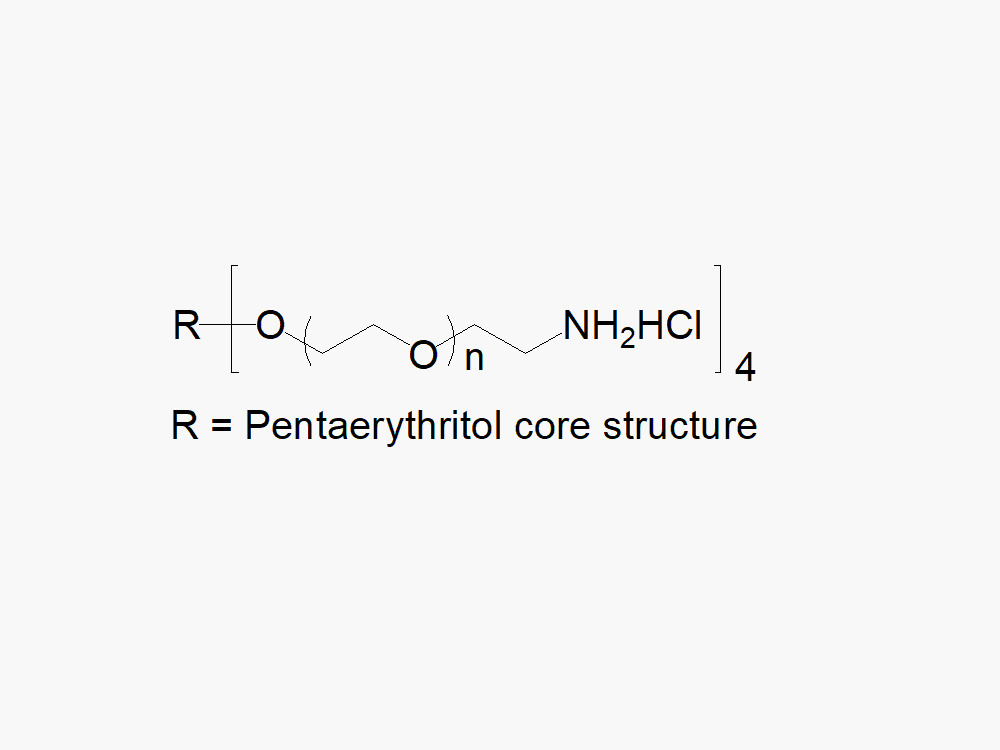
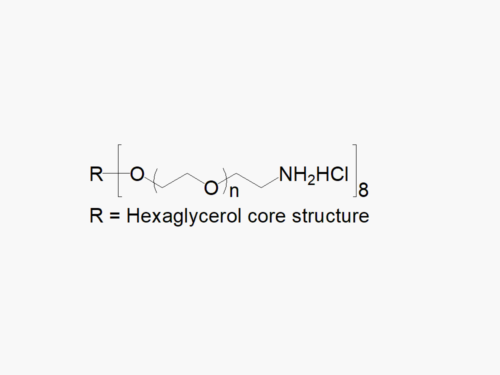
4arm PEG Amine, HCl Salt
$50.00 – $2,400.00
Description
4arm PEG Amine, HCl Salt, reagent with superior quality specification of ≥ 95% Substitution.
JenKem Technology’s 4arm PEG Amine derivatives can be cross-linked into PEG hydrogels. The HCl salt provides stability to the solid form of 4arm amine PEG. PEG hydrogels have a variety of applications in medical devices and regenerative medicine, and are especially of interest for controlled release of drugs, for 2D and 3D cell culture, and for wound sealing and healing. JenKem Technology’s 4arm PEGs are synthesized by ethoxylation of pentaerythritol. The number of ethylene oxide units in the PEG chain may not be equal for all arms. The total molecular weight reported for the JenKem multi-arm PEGs is the sum of the PEG molecular weights of each arm.
4arm NH2 star PEG products with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
Note: Starting July 2016, 4ARM-NH2HCl is the new item number of the product 4ARM-NH2 (MW 5000 (4ARM-NH2 5000), MW 10000 (4ARM-NH2-10K), MW20000 (4ARM-NH2-20K) and MW40000 (4ARM-NH2-40K).
References:
- Zou, Z., et al., Injectable antibacterial tissue-adhesive hydrogel based on biocompatible o-phthalaldehyde/amine crosslinking for efficient treatment of infected wounds, Biomaterials, 301, 2023.
- Zhao, J., et al., A triple crosslinked micelle-hydrogel lacrimal implant for localized and prolonged therapy of glaucoma, European Journal of Pharmaceutics and Biopharmaceutics, V. 185, 2023, P. 44-54.
- Si, X., et al., Comprehensive evaluation of biopolymer immune implants for peritoneal metastasis carcinoma therapy, Journal of Controlled Release, V. 353, 2023, P. 289-302.
- Zhao, D., et al., Rapidly Thermoreversible and Biodegradable Polypeptide Hydrogels with Sol–Gel–Sol Transition Dependent on Subtle Manipulation of Side Groups. Biomacromolecules. 2021.
- Sharma, S, et al., The effects of processing variables on electrospun poly (ethylene glycol) fibrous hydrogels formed from the thiol‐norbornene click reaction. Journal of Applied Polymer Science. 2021, 138(32):50786.
- Schirmer, L, et al, Chemokine‐Capturing Wound Contact Layer Rescues Dermal Healing. Advanced Science. 2021, 2100293.
Kim, S, et al., In situ mechanical reinforcement of polymer hydrogels via metal-coordinated crosslink mineralization. Nature communications. 2021, 12(1):1-0. - Song, J, et al, Influence of Poly (ethylene glycol) Molecular Architecture on Particle Assembly and Ex Vivo Particle–Immune Cell Interactions in Human Blood. ACS nano. 2021.
- Baker, A., et al., Stable oxime-crosslinked hyaluronan-based hydrogel as a biomimetic vitreous substitute, Biomaterials, 2021, V. 271.
- Lee, Y.Y., et al, Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm, Biomaterials, 2021, 267, 120389.
- Ding, Y. et al, Tethering transforming growth factor β1 to soft hydrogels guides vascular smooth muscle commitment from human mesenchymal stem cells, Acta Biomaterialia, 2020, V. 105, P. 68-77.
- Newland, B., et al., Focal drug administration via heparin-containing cryogel microcarriers reduces cancer growth and metastasis, Carbohydrate Polymers, 2020, 245, 116504.
- Newland, B., et al., Static and dynamic 3D culture of neural precursor cells on macroporous cryogel microcarriers, MethodsX, 2020, V.7.
- Zhan, H., et al., A Hybrid Peptide Amphiphile Fiber PEG Hydrogel Matrix for 3D Cell Culture, Advanced Functional Materials, 2019.
- Bizeau, J., et al., Synthesis and characterization of hyaluronic acid coated manganese dioxide microparticles that act as ROS scavengers, Colloids and Surfaces B: Biointerfaces, 2017, 159, P. 30-38.
- Sharma, S., et al., A photoclickable peptide microarray platform for facile and rapid screening of 3-D tissue microenvironments, Biomaterials, 2017, 143, P. 17-28.
- Tardy, B.L., et al., Formation of Polyrotaxane Particles via Template Assembly. Biomacromolecules, 2017.
- Hu, J., et al., A thermo-degradable hydrogel with light-tunable degradation and drug release, Biomaterials, 2017, 112, p. 133-140.
- Aliperta, R., et al., Cryogel-supported stem cell factory for customized sustained release of bispecific antibodies for cancer immunotherapy, Scientific Reports, 2017, 7.
- Li Y, et al., Water-dispersible graphene/amphiphilic pyrene derivative nanocomposite: High AuNPs loading capacity for CEA electrochemical immunosensing, Sensors and Actuators B: Chemical, 2017.
- Racine, L., et al., Design of interpenetrating chitosan and poly (ethylene glycol) sponges for potential drug delivery applications, Carbohydrate Polymers, 2017, 170:166-75.
- Rehmann, M., et al., Tuning microenvironment modulus and biochemical composition promotes human mesenchymal stem cell tenogenic differentiation, J. Biomed. Mater. Res. A, 2016.
- Borg, D.J., et al., Macroporous biohybrid cryogels for co-housing pancreatic islets with mesenchymal stromal cells, Acta Biomaterialia, 2016.
- Yesilyurt, V., et al., Injectable self‐healing glucose‐responsive hydrogels with pH‐regulated mechanical properties, Advanced Materials, 2016, 28(1), p. 86-91.
- Henise, J.. et al., Biodegradable Tetra-PEG Hydrogels as Carriers for a Releasable Drug Delivery System, Bioconjugate Chem., 2015, 26 (2), pp 270–278.
- Jonker, A. M., et al., A Fast and Activatable Cross-Linking Strategy for Hydrogel Formation, Advanced Materials, 2015, 27(7): 1235-1240.
- Zhang, N., et al., Magnetic Nanocomposite Hydrogel for Potential Cartilage Tissue Engineering: Synthesis, Characterization, and Cytocompatibility with Bone Marrow Derived Mesenchymal Stem Cells, ACS Applied Materials & Interfaces, 2015, 7 (37), 20987-20998.
- Truong, V.X., et al., Photodegradable Gelatin-Based Hydrogels Prepared by Bioorthogonal Click Chemistry for Cell Encapsulation and Release, Biomacromolecules, 2015, 16 (7), 2246-2253.
- Learsch, R., Engineering mechanical dissipation in solid poly(ethylene glycol) hydrogels with bio-inspired metal-coordinate crosslinks, 2015, MIT.
- Cheng, X.Q., et al., Nanofiltration membrane achieving dual resistance to fouling and chlorine for “green” separation of antibiotics, Journal of Membrane Science, 2015, V. 493, P. 156-166.
- Truong, V. X., et al., Nitrile Oxide-Norbornene Cycloaddition as a Bioorthogonal Crosslinking Reaction for the Preparation of Hydrogels, Macromol. Rapid Commun., 2015, 36: 1729–1734.
- Houbenov, N., et al., Halogen-bonded mesogens direct polymer self-assemblies up to millimetre length scale, Nature Communications, 2014, 5:4043.
- Myers, B.K., et al., The characterization of dendronized poly(ethylene glycol)s and poly(ethylene glycol) multi-arm stars using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, Analytica Chimica Acta, 2014, 808(0): p. 175-189.
- Hao, Y., et al., Visible light cured thiol-vinyl hydrogels with tunable degradation for 3D cell culture. Acta Biomaterialia, 2014, 10(1): p. 104-114.
- Xu, J., E. Feng, and J. Song, Bioorthogonally Cross-Linked Hydrogel Network with Precisely Controlled Disintegration Time over a Broad Range. Journal of the American Chemical Society, 2014, 136(11): p. 4105-4108.
- McKinnon, D.D., et al., Bis-Aliphatic Hydrazone-Linked Hydrogels Form Most Rapidly at Physiological pH: Identifying the Origin of Hydrogel Properties with Small Molecule Kinetic Studies. Chemistry of Materials, 2014, 26(7): p. 2382-2387.
- Ki, C.S., et al., Thiol-ene hydrogels as desmoplasia-mimetic matrices for modeling pancreatic cancer cell growth, invasion, and drug resistance, Biomaterials, 2014, 35(36), p: 9668-9677.
- McKinnon, D.D., et al., Design and Characterization of a Synthetically Accessible, Photodegradable Hydrogel for User-Directed Formation of Neural Networks, Biomacromolecules, 2014, 15, 2808−2816.
- Jonker, A.M., et al., A Fast and Activatable Cross-Linking Strategy for Hydrogel Formation, Advanced Materials, 2014, 27(7), 1235-1240.
- Shih, H., et al.,Visible-Light-Mediated Thiol-Ene Hydrogelation Using Eosin-Y as the Only Photoinitiator, Macromolecular Rapid Communications, 2013, 34(3): 269-273.
- , G.W., et al., Hydrogel drug delivery system with predictable and tunable drug release and degradation rates, PNAS, 2013, 110(6) 2318-2323.
- Maitz, M.F., et al., Bio-responsive polymer hydrogels homeostatically regulate blood coagulation, Nature Commun, 2013, 4.
- Kretlow, James D., Biomaterial-based strategies for craniofacial tissue engineering, Doctoral Thesis, Rice University, 2010.
- Murphy, J.L., et al., Adhesive Performance of Biomimetic Adhesive-Coated Biologic Scaffolds, Biomacromolecules, 2010, 11(11), pp 2976–2984.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.