PEG products with additional MW may be made to order, please contact us for details
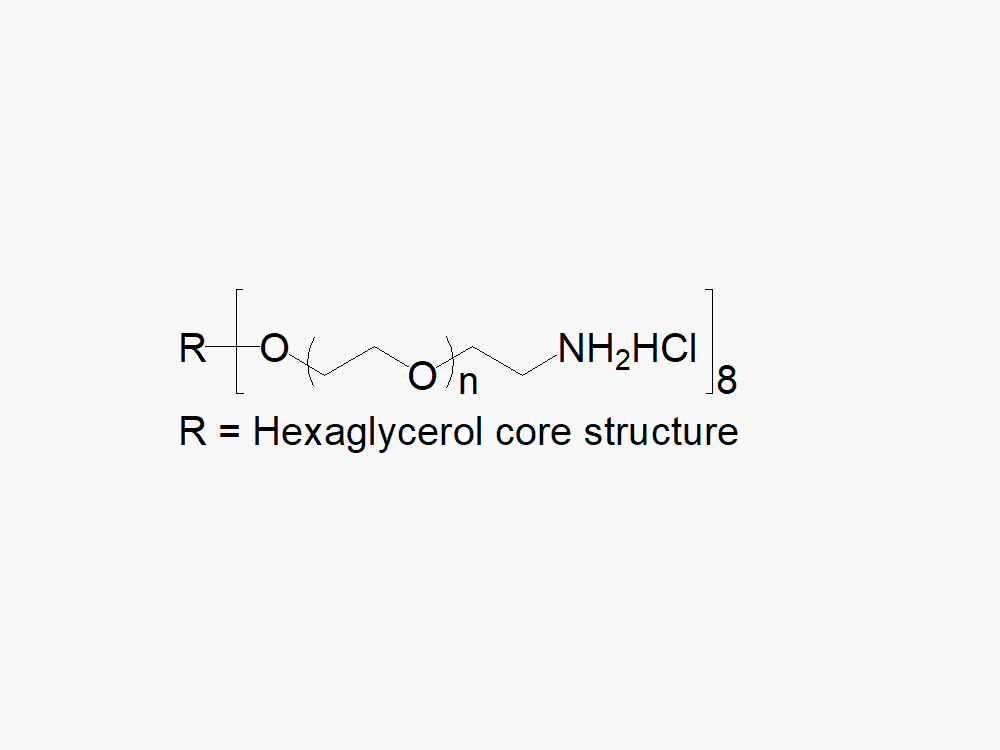
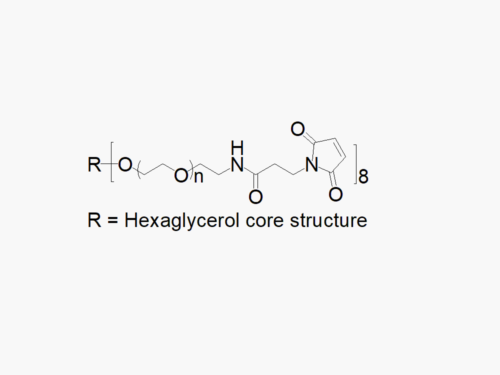
8arm PEG Amine (hexaglycerol), HCl Salt
$50.00 – $2,000.00
Description
8arm PEG Amine (hexaglycerol), HCl Salt, reagent with superior quality specification of ≥ 95% Substitution.
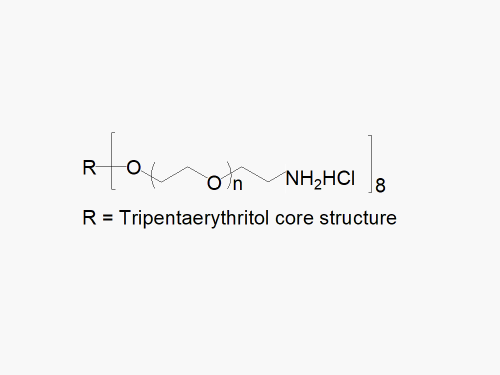
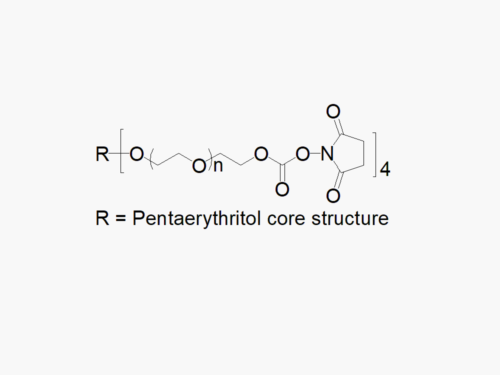
8arm PEG Amine (hexaglycerol) HCl salt derivatives can be crosslinked into PEG hydrogels. PEG hydrogels have a variety of applications in medical devices and regenerative medicine, and are especially of interest for controlled release of drugs, for 2D and 3D cell culture, and for wound sealing and healing. JenKem Technology’s 8 arm PEGs are synthesized by ethoxylation of tripentaerythritol (8ARM PEG(TP)) or hexaglycerol (8ARM PEG). The number of ethylene oxide units in the PEG chain may not be equal for all arms. The total molecular weight reported for the JenKem multi-arm PEGs is the sum of the PEG molecular weights of each arm.
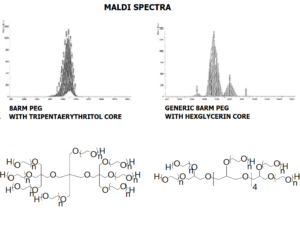
JenKem Technology also provides 8ARM(TP)-PEG hydroxyl raw materials and PEG derivatives with tripentaerythritol core. 8ARM(TP)-PEGs with tripentaerythritol core have a higher purity as evidenced by MALDI compared with the generic 8ARM-PEGs with a hexaglycerin core.
Multi-arm star PEG products with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
References:
- Bhatta, R., et al., T cell-responsive macroporous hydrogels for in situ T cell expansion and enhanced antitumor efficacy, Biomaterials, V. 293, 2023.
- Schroeder, M. E., et al., Osteopontin activity modulates sex‐specific calcification in engineered valve tissue mimics, Bioengineering & translational medicine 2023, 8.1, e10358.
- Batan, D, et al., Hydrogel cultures reveal Transient Receptor Potential Vanilloid 4 regulation of myofibroblast activation and proliferation in valvular interstitial cells. The FASEB Journal. 2022.
- Yu, Y, et al., A 3D printed mimetic composite for the treatment of growth plate injuries in a rabbit model. NPJ Regenerative Medicine. 2022.
- Chauhan, N., et al., Dexamethasone-loaded, injectable pullulan-poly(ethylene glycol) hydrogels for bone tissue regeneration in chronic inflammatory conditions, Materials Science and Engineering: C, 2021, V. 130
- Schoonraad, SA, et al., The Effects of Stably Tethered BMP-2 on MC3T3-E1 Preosteoblasts Encapsulated in a PEG Hydrogel. Biomacromolecules. 2021, 22(3):1065-79.
- Caldwell, AS, et al, Mesenchymal stem cell‐inspired microgel scaffolds to control macrophage polarization. Bioengineering & Translational Medicine. 2021, 6(2):e10217.
- Song, J, et al, Influence of Poly (ethylene glycol) Molecular Architecture on Particle Assembly and Ex Vivo Particle–Immune Cell Interactions in Human Blood. ACS nano. 2021.
- Schroeder, M. E., et al., Collagen networks within 3D PEG hydrogels support valvular interstitial cell matrix mineralization, Acta Biomaterialia, 2021, V. 119, P. 197-210.
- Fakhouri, A. S., et al., High-Throughput Three-Dimensional Hydrogel Cell Encapsulation Assay for Measuring Matrix Metalloproteinase Activity, Assay and drug development technologies, 2019, 17(3): 100-115.
- Tang, J., et al., Quantitative and high drug loading of self-assembled prodrug with defined molecular structures for effective cancer therapy, Journal of Controlled Release, 2019, 307, p. 90-97.
- Schneider, M.C., et al., An In Vitro and In Vivo Comparison of Cartilage Growth in Chondrocyte-Laden Matrix Metalloproteinase-Sensitive Poly (Ethylene Glycol) Hydrogels with Localized Transforming Growth Factor β3, Acta biomaterialia, 2019.
- Armstrong, J.P., et al., Spatiotemporal quantification of acoustic cell patterning using Voronoï tessellation, Lab on a Chip, 2019.
- Rao, V.V., et al., Rescuing mesenchymal stem cell regenerative properties on hydrogel substrates post serial expansion, Bioengineering & translational medicine, 2019.
- Aziz, A.H., et al., The effects of dynamic compressive loading on human mesenchymal stem cell osteogenesis in the stiff layer of a bilayer hydrogel, Journal of tissue engineering and regenerative medicine, 2019.
- Aziz, A.H., et al., A comparison of hMSC osteogenesis in PEG hydrogels as a function of MMP‐sensitive crosslinker and crosslink density in chemically‐defined medium, Biotechnology and Bioengineering, 2019.
- Lee, J., et al., Glucose‐Responsive Trehalose Hydrogel for Insulin Stabilization and Delivery, Macromolecular bioscience, 2018, p.1700372.
- Carles-Carner, M., et al., The effects of hydroxyapatite nanoparticles embedded in a MMP-sensitive photoclickable PEG hydrogel on encapsulated MC3T3-E1 pre-osteoblasts, Biomedical Materials, 2018, 13(4), p.045009.
- Ovadia, E.M., et al., Designing well-defined photopolymerized synthetic matrices for three-dimensional culture and differentiation of induced pluripotent stem cells, Biomaterials science, 2018.
- Li, H., et al., Preparation of photo-responsive poly(ethylene glycol) microparticles and their influence on cell viability, Journal of Colloid and Interface Science, 2018, V. 514, P. 182-189.
- Tardy, B.L., et al., Formation of Polyrotaxane Particles via Template Assembly. Biomacromolecules, 2017.
- Guo, C., et al., Bio-orthogonal conjugation and enzymatically triggered release of proteins within multi-layered hydrogels, Acta Biomaterialia, 2017.
- Schneider, M.C., et al., Characterization of the chondrocyte secretome in photoclickable poly (ethylene glycol) hydrogels, Biotechnology and Bioengineering, 2017.
- Aziz, A.H., et al., Mechanical characterization of sequentially layered photo-clickable thiol-ene hydrogels, Journal of the Mechanical Behavior of Biomedical Materials, 2017, V. 65, p. 454-465.
- Braunger, J.A., et al., Interactions between circulating nanoengineered polymer particles and extracellular matrix components in vitro. Biomaterials Science, 2017.
- Liu, Y., et al., Trehalose Glycopolymer Enhances Both Solution Stability and Pharmacokinetics of a Therapeutic Protein, Bioconjugate Chemistry, 2017.
- Shih, H., Improving gelation efficiency and cytocompatibility of visible light polymerized thiol-norbornene hydrogels via addition of soluble tyrosine, Biomaterials Science, 2017, 5(3), 589-99.
- Chu, S., et al., Understanding the Spatiotemporal Degradation Behavior of Aggrecanase-Sensitive Poly (ethylene glycol) Hydrogels for use in Cartilage Tissue Engineering, Tissue Engineering, 2017.
- Shin, D.S., et al., Synthesis of microgel sensors for spatial and temporal monitoring of protease activity, ACS Biomaterials Science & Engineering, 2017.
- Jeon, O., et al., Dual-crosslinked hydrogel microwell system for formation and culture of multicellular human adipose tissue-derived stem cell spheroids. Journal of Materials Chemistry B, 2016, 4(20), 3526-33.
- Ma, Z., et al., Folate‐Conjugated Polylactic Acid–Silica Hybrid Nanoparticles as Degradable Carriers for Targeted Drug Delivery, On‐Demand Release and Simultaneous Self‐Clearance, ChemPlusChem, 2016.
- Maisonneuve, B. G. C., et al., Effects of Synthetic Biomacromolecule Addition on the Flow Behavior of Concentrated Mesenchymal Cell Suspensions, Biomacromolecules, 2015, 16(1), 275-283.
- Hennig, R., et al., Branched Polymer–Drug Conjugates for Multivalent Blockade of Angiotensin II Receptors, Molecular Pharmaceutics, 2015, 12 (9), 3292-3302.
- Skaalure, S.C., et al., An Enzyme-Sensitive PEG Hydrogel Based on Aggrecan Catabolism for Cartilage Tissue Engineering. Advanced Healthcare Materials, 2015, 4, 420–431.
- Sarfare, S., et al., Biocompatibility of a Synthetic Biopolymer for the Treatment of Rhegmatogenous
Retinal Detachment, J Clin Exp Ophthalmol, 2015, 6, 475. - Frith, J.E., et al., Effects of bound versus soluble pentosan polysulphate in PEG/HA-based hydrogels tailored for intervertebral disc regeneration. Biomaterials, 2014, 35(4), p. 1150-1162.
- Jeon, O., et al., Single and dual crosslinked oxidized methacrylated alginate/PEG hydrogels for bioadhesive applications. Acta Biomaterialia, 2014, 10(1), p. 47-55.
- McKinnon, D.D., et al., Design and Characterization of a Synthetically Accessible, Photodegradable Hydrogel for User-Directed Formation of Neural Networks, Biomacromolecules, 2014, 15, 2808−2816.
- Amoozgar, Z., et al., Dual-layer surface coating of PLGA-based nanoparticles provides slow-release drug delivery to achieve metronomic therapy in a paclitaxel-resistant murine ovarian cancer model, Biomacromolecules, 2014, 15(11), 4187-94.
- Chung, J., et al., Modular Multi-enzyme Cascade Process Using Highly Stabilized Enzyme Microbeads, Green Chem., 2014, 16, 1163-1167.
- McKinnon, D.D., Process Extension from Embryonic Stem Cell-Derived Motor Neurons through Synthetic Extracellular Matrix Mimics, Univ of Colorado at Boulder, 2014, 3635878.
- Cui, J., et al., Super-Soft Hydrogel Particles with Tunable Elasticity in a Microfluidic Blood Capillary Model, Advanced Materials, 2014, 26(43), 7295-7299.
- Gandavarapu, N. R., et al., Osteogenic differentiation of human mesenchymal stem cells on α5 integrin binding peptide hydrogels is dependent on substrate elasticity, Biomaterials Science, 2014, 2(3), 352-361.
- McKinnon, D.D., et al., Measuring cellular forces using bis-aliphatic hydrazone crosslinked stress-relaxing hydrogels, Soft Matter, 2014, 10, 9230.
- Strehin, I., et al., Hydrogels Formed by Oxo-ester Mediated Native Chemical Ligation, Biomater Sci., 2013, 1(6), 603–613.
- Barrett, D. G., et al., pH-Based Regulation of Hydrogel Mechanical Properties Through Mussel-Inspired Chemistry and Processing, Adv Funct Mater, 2013, 23(9), 1111-1119.
- Chung, J., et al., Magnetic-separable robust microbeads using a branched polymer for stable enzyme immobilization, Reactive and Functional Polymers, 2013, 73:1, P. 39-45.
- Jeon, O., et al., Regulation of Stem Cell Fate in a Three‐Dimensional Micropatterned Dual‐Crosslinked Hydrogel System, Advanced functional materials, 2013, 23.38, 4765-4775.
- Giorgi, M.E., et al., Improved bioavailability of inhibitors of Trypanosoma cruzi trans-sialidase: PEGylation of lactose analogs with multiarm polyethyleneglycol, Glycobiology, 2012, 22(10), p. 1363-1373.
- Zhou, J., et al., Real-time detection of implant-associated neutrophil responses using a formyl peptide receptor-targeting NIR nanoprobe, International Journal of Nanomedicine, 2012, 7 2057–2068.
- Christman, K.L., et al., Surface Patterning for Generating Defined Nanoscale Matrices, Stem Cells for Myocardial Regeneration, 2010, v. 660, p. 255-263.
- Tan, H., et al., Novel Multi-arm PEG-based Hydrogels for Tissue Engineering, Journal of biomedical materials research Part A., 2010, 92(3), 979-987.
Click here to download the MSDS
Note: Starting July 2016, 8ARM-NH2HCl is the new name of the product 8ARM-NH2 (MW 10K (8ARM-NH2-10K), MW 20K (8ARM-NH2-20K) and MW 40K (8ARM-NH2-40K)).
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.