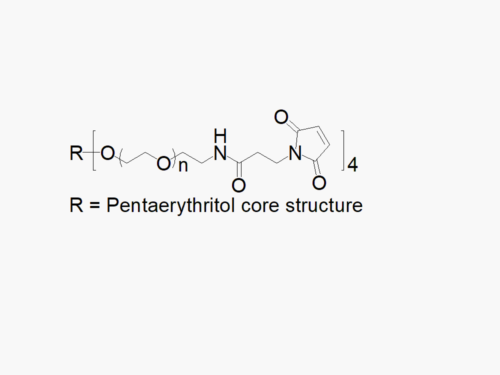
PEG products with additional MW may be made to order, please contact us for details
4arm PEG Succinimidyl Glutarate
$80.00 – $720.00
Description
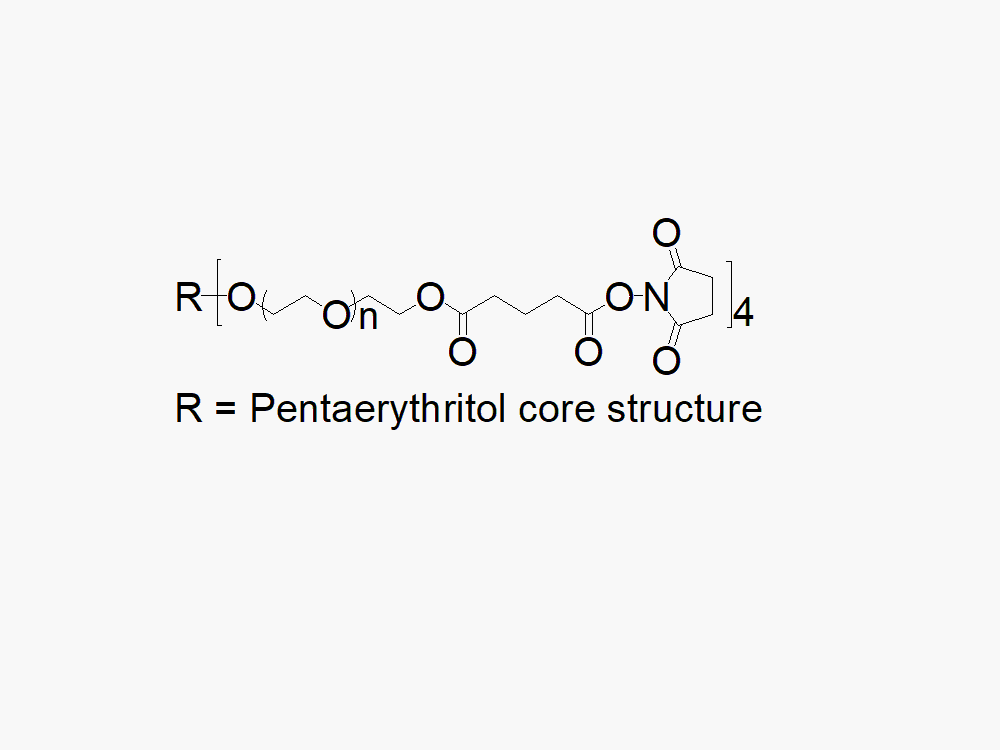
4arm PEG Succinimidyl Glutarate reagent with superior quality specification of ≥ 95% Substitution.
JenKem Technology’s 4arm PEG Succinimidyl Glutarate (a.k.a., 4S-StarPEG) derivatives can be crosslinked into degradable PEG hydrogels. 4arm PEG Succinimidyl Glutarate is a cleavable labile PEG linker. The cleavable ester linker between PEG and the NHS ester enables the feature of “degradable” hydrogels. PEG hydrogels have a variety of applications in medical devices and regenerative medicine, and are especially of interest for controlled release of drugs, for 2D and 3D cell culture, and for wound sealing and healing. JenKem Technology’s 4 arm PEGs are synthesized by ethoxylation of pentaerythritol. The number of ethylene oxide units in the PEG chain may not be equal for all arms. The total molecular weight reported for the JenKem multi-arm PEGs is the sum of the PEG molecular weights of each arm.
Multi-arm star PEG products with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Pugliese, E., et al., Development of three-layer collagen scaffolds to spatially direct tissue-specific cell differentiation for enthesis repair, Materials Today Bio, 19, 2023.
- Giliomee, J., et al., Investigation of the 3D Printability of Covalently Cross-Linked Polypeptide-Based Hydrogels. ACS omega. 2022; 7(9).
- Pereira, D.R., et al., Macromolecular modulation of a 3D hydrogel construct differentially regulates human stem cell tissue-to-tissue interface, Biomaterials Advances, 2022, 133.
- Wang, Y., et al., Efficacy and safety of scleral crosslinking using poly (ethylene glycol) ether tetrasuccinimidyl glutarate for form-deprivation myopia progression in rabbits. RSC Advances. 2021, 11(50):31746-55.
- Wu, Z., et al., In the quest of the optimal chondrichthyan for the development of collagen sponges for articular cartilage, Journal of Science: Advanced Materials and Devices, 2021.
- Wu, Z., et al., The Influence of Bloom Index, Endotoxin Levels and Polyethylene Glycol Succinimidyl Glutarate Crosslinking on the Physicochemical and Biological Properties of Gelatin Biomaterials. Biomolecules. 2021, 11(7):1003.
- Giliomee, J, et al., Evaluation of Composition Effects on the Physicochemical and Biological Properties of Polypeptide-Based Hydrogels for Potential Application in Wound Healing. Polymers. 2021, 13(11):1828.
- Wu, Z., et al., In the quest of the optimal tissue source (porcine male and female articular, tracheal and auricular cartilage) for the development of collagen sponges for articular cartilage, Biomedical Engineering Advances, 2021, V. 1.
- Na, K.-S., et al., Effect of mesenchymal stromal cells encapsulated within polyethylene glycol-collagen hydrogels formed in situ on alkali-burned corneas in an ex vivo organ culture model, Cytotherapy, 2021, V. 23 (6), P. 500-509.
- Yu, B., et al., A designed supramolecular cross-linking hydrogel for the direct, convenient, and efficient administration of hydrophobic drugs, International Journal of Pharmaceutics, 2020, V. 578.
- Delgado, L., et al., Collagen cross-linking–Biophysical, biochemical and biological response analysis. Tissue Engineering. 2017.
- Lotz, C., et al., A Crosslinked Collagen Hydrogel Matrix Resisting Contraction to Facilitate Full-thickness Skin Equivalents, ACS Applied Materials & Interfaces, 2017.
- Inostroza-Brito, K.E., et al., Cross-linking of a Biopolymer-Peptide Co-Assembling System, Acta Biomaterialia, 2017.
- Corrales, R., et al., Mechanical modulation of a human plasma based skin scaffold via reactive multi-arm polyethylene glycols, Biomecanica, 2016, 24, pp 14-23.
- Kontturi, L.S., et al., Encapsulated cells for long-term secretion of soluble VEGF receptor 1: Material optimization and simulation of ocular drug response, European Journal of Pharmaceutics and Biopharmaceutics, 2015, V. 95, B, Pages 387-397.
- Sanami, M., et al., The influence of poly(ethylene glycol) ether tetrasuccinimidyl glutarate on the structural, physical, and biological properties of collagen fibers, J. Biomed. Mater. Res., 2015.
- Fontana, G., et al., Three-Dimensional Microgel Platform for the Production of Cell Factories Tailored for the Nucleus Pulposus, Bioconjugate Chem., 2015, 26 (7), pp 1297–1306.
- Sanami, M., et al., Biophysical and biological characterisation of collagen/resilin-like protein composite fibres, Biomedical Materials, 2015, 10:6.
- Kontturi, L.S., et al., An injectable, in situ forming type II collagen/hyaluronic acid hydrogel vehicle for chondrocyte delivery in cartilage tissue engineering, Drug delivery and translational research, 2014, 4(2):149-58.
- Thomas, D., et al., A shape-controlled tuneable microgel platform to modulate angiogenic paracrine
responses in stem cells, Biomaterials, 2014, 35(31):8757-8766. - Grover, G.N., et al., Myocardial Matrix-Polyethylene Glycol Hybrid Hydrogels for Tissue Engineering, Nanotechnology, 2014, 25(1):014011.
- Michael Monaghan, et al., A Collagen-based Scaffold Delivering Exogenous MicroRNA-29B to Modulate Extracellular Matrix Remodeling, Molecular Therapy, 2014, 22 (4), p: 786–796.
- Fontana, G., et al., Microgel Microenvironment Primes Adipose-Derived Stem Cells Towards an NP Cells-Like Phenotype. Advanced Healthcare Materials, 2014, 3: 2012–2022.
- Brunette, M., et al., Inducible Nitric Oxide Releasing Poly-(Ethylene Glycol)-Fibrinogen Adhesive Hydrogels for Tissue Regeneration, MRS Spring Meeting, 2013.
- Sargeant, T.D., et al., An in situ forming collagen–PEG hydrogel for tissue regeneration. Acta Biomaterialia, 2012. 8(1): p. 124-132.
- Rane, A.A., Understanding mechanisms by which injectable biomaterials affect cardiac function postmyocardial infarction, UC San Diego, 2012.
- Collin, E.C., et al., An injectable vehicle for nucleus pulposus cell-based therapy, Biomaterials, 2011, 32(11), p: 2862-2870.
- Collin, E., et al., Injectable Type II Collagen-Hyluronan Hydrogel As Reservoir System For Nucleus Pulposus Regeneration, European Cells and Materials, 2010, 20(2).
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.