PEG products with additional MW may be made to order, please contact us for details
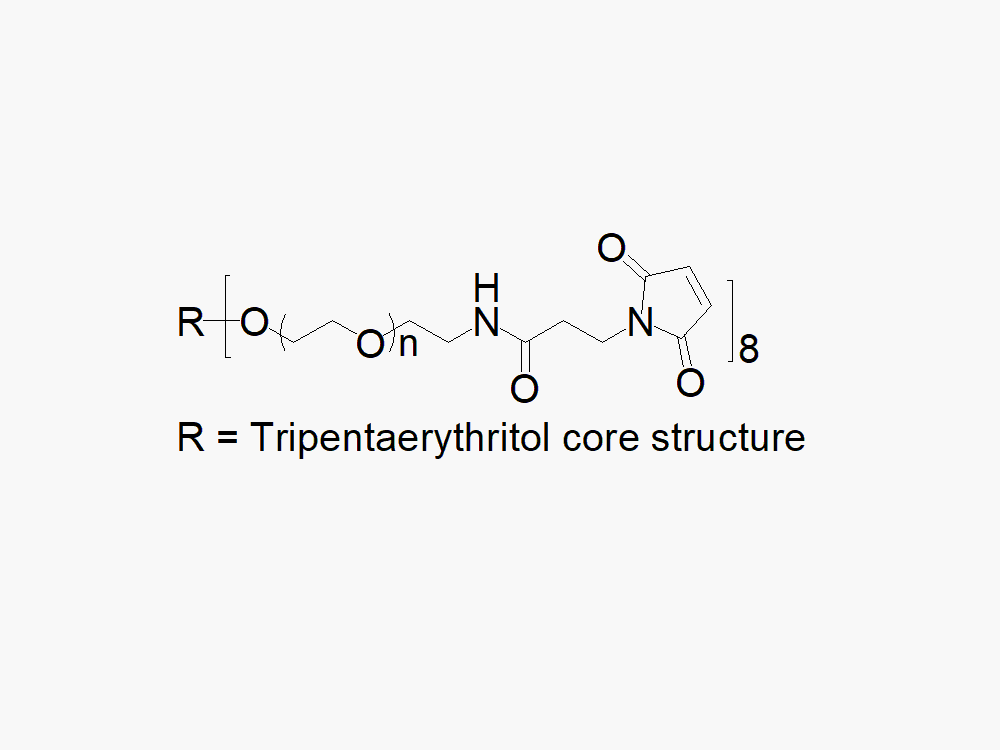
8arm PEG Maleimide (tripentaerythritol)
$130.00 – $1,120.00
Description
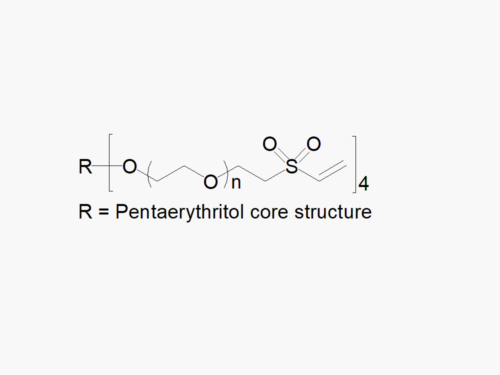
8arm PEG Maleimide (tripentaerythritol) with superior quality specification of >90% Substitution.
JenKem Technology’s 8arm PEG Maleimide (tripentaerythritol) derivatives can be crosslinked into PEG hydrogels. PEG hydrogels have a variety of applications in medical devices and regenerative medicine, and are of interest for controlled release of drugs, for 2D and 3D cell culture, and for wound sealing and healing. JenKem Technology’s 8arm (TP) PEGs are synthesized by ethoxylation of tripentaerythritol. 8ARM(TP)-PEG polymers with tripentaerythritol core have a higher purity as evidenced by MALDI compared with the generic 8ARM-PEG polymers with a hexaglycerin core.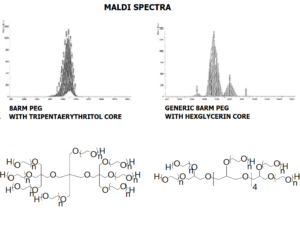
The number of ethylene oxide units in the PEG chain may not be equal for all arms. The total molecular weight reported for the JenKem multi-arm PEGs is the sum of the PEG molecular weights of each arm.
Multi-arm star PEG products with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
Bulk PEGs and GMP PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Bekdemir, A., et al., Ionic Liquid‐Mediated Transdermal Delivery of Thrombosis‐Detecting Nanosensors. Advanced Healthcare Materials. 2022.
- Shatz, W., et al., Identification and characterization of an octameric PEG-protein conjugate system for intravitreal long-acting delivery to the back of the eye. PLoS One, 2019, 14(6), p.e0218613.
- Manzoli, V., et al., Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol, American Journal of Transplantation, 2018, 18(3):590-603.
- Dudani, J. S., et al., Sustained-Release Synthetic Biomarkers for Monitoring Thrombosis and Inflammation Using Point-of-Care Compatible Readouts. Adv. Funct. Mater., 2016.
- Lu, H.D., et al., Injectable shear-thinning hydrogels engineered with a self-assembling Dock-and-Lock mechanism. Biomaterials, 2012, 33(7): p. 2145-2153.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.