PEG products with additional MW may be made to order, please contact us for details
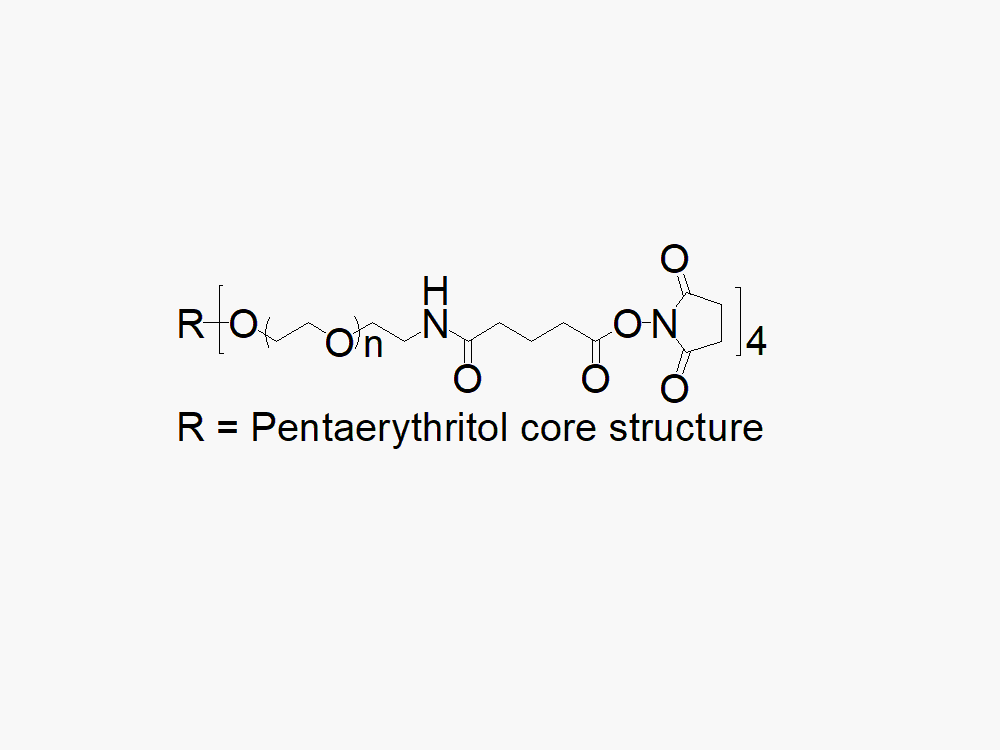
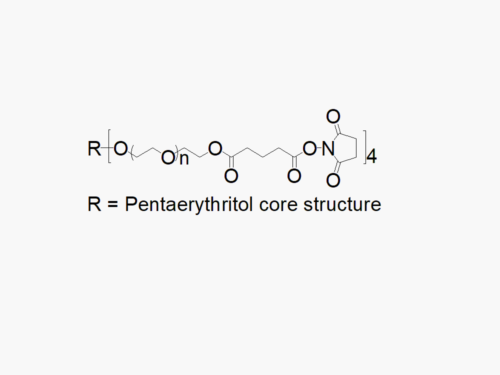
4arm PEG Succinimidyl Glutaramide
$120.00 – $960.00
Description
4arm PEG Succinimidyl Glutaramide reagent with superior quality specification of ≥ 95% Substitution.
JenKem Technology’s 4arm PEG Succinimidyl Glutaramide derivatives can be cross-linked into PEG hydrogels. 4arm SGA has a stable NHS linker and offers a longer half-life than 4arm SCM. PEG hydrogels have a variety of applications in medical devices and regenerative medicine, and are especially of interest for controlled release of drugs, for 2D and 3D cell culture, and for wound sealing and healing. JenKem Technology’s 4 arm PEGs are synthesized by ethoxylation of pentaerythritol. The number of ethylene oxide units in the PEG chain may not be equal for all arms. The total molecular weight reported for the JenKem multi-arm PEGs is the sum of the PEG molecular weights of each arm.
Multi-arm star PEG products with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Buwalda, S.J., et al., Ultrafast in situ forming poly (ethylene glycol)-poly (amido amine) hydrogels with tunable drug release properties via controllable degradation rates, European Journal of Pharmaceutics and Biopharmaceutics, 2019, 139:232-9.
- Li, F., et al., Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells, Acta Biomaterialia, 2018, V. 77, P. 48-62.
- Suarez, S.L., et al., Intramyocardial injection of hydrogel with high interstitial spread does not impact action potential propagation, Acta Biomaterialia, 2015, V. 26, P. 13-22.
- Learsch, R., Engineering mechanical dissipation in solid poly(ethylene glycol) hydrogels with bio-inspired metal-coordinate crosslinks, MIT, 2015.
- Rane, A.A., Understanding mechanisms by which injectable biomaterials affect cardiac function postmyocardial infarction, UC San Diego, 2012.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.