PEG products with additional MW may be made to order, please contact us for details
4arm PEG Acrylate
$80.00 – $640.00
Description
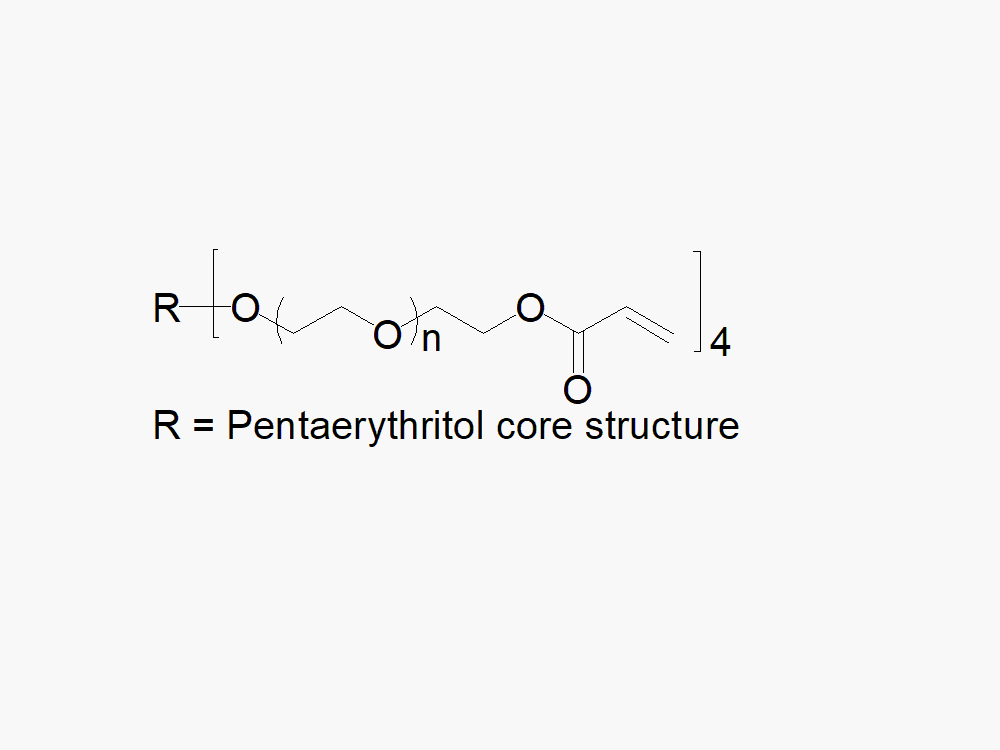
4arm PEG Acrylate reagent with superior quality specification of ≥ 95% Substitution.
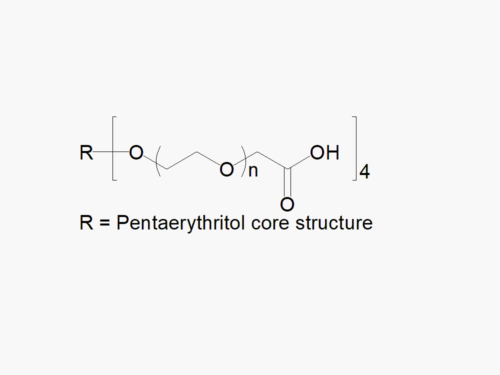
JenKem Technology’s 4arm PEG Acrylate derivatives can be cross-linked into PEG hydrogels. PEG hydrogels have a variety of applications in medical devices and regenerative medicine, and are especially of interest for controlled release of drugs, for 2D and 3D cell culture, and for wound sealing and healing. JenKem Technology’s 4 arm PEGs are synthesized by ethoxylation of pentaerythritol. The number of ethylene oxide units in the PEG chain may not be equal for all arms. The total molecular weight reported for the JenKem multi-arm PEGs is the sum of the PEG molecular weights of each arm.
Multi-arm star PEG products with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom PEG synthesis. Please inquire at tech@jenkemusa.com about pricing and availability of custom PEGs.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Ma, X., et al., Multifunctional injectable hydrogel promotes functional recovery after stroke by modulating microglial polarization, angiogenesis and neuroplasticity, Chemical Engineering Journal, V. 464, 2023.
- Giliomee, J., et al., Investigation of the 3D Printability of Covalently Cross-Linked Polypeptide-Based Hydrogels. ACS omega. 2022.
- Ma, Z., et al., 3D bioprinting of proangiogenic constructs with induced immunomodulatory microenvironments through a dual cross-linking procedure using laponite incorporated bioink, Composites Part B: Engineering, 2022, 229.
- Huang, Y., et al., Structural aspects controlling the mechanical and biological properties of tough, double network hydrogels. Acta Biomaterialia, 2022.
- Sun, X., et al., Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus, Bioactive Materials, 6(3), 2021, P. 757-769.
- Uppal, G., et al., Tissue Failure Propagation as Mediated by Circulatory Flow, Biophysical Journal, 2020, 119(12), P. 2573-2583.
- Kwak, H., et al., Colorimetric assay of tyrosinase inhibition using melanocyte laden hydrogel fabricated by digital light processing printing, Journal of Industrial and Engineering Chemistry, 2020, 84, p. 252-259.
- McKee, C., et al., Mesenchymal stem cells transplanted with self-assembling scaffolds differentiated to regenerate nucleus pulposus in an ex vivo model of degenerative disc disease, Applied Materials Today, 2020, V. 18.
- Day, J.R., et al., The impact of functional groups of poly(ethylene glycol) macromers on the physical properties of photo-polymerized hydrogels and the local inflammatory response in the host, Acta Biomaterialia, 2018, Vol. 67, P. 42-52.
- Imaninezhad, M., et al, Cell Microencapsulation in Polyethylene Glycol Hydrogel Microspheres Using Electrohydrodynamic Spraying, Methods Mol Biol, 2017.
- Jain, E., et al., Control of gelation, degradation and physical properties of polyethylene glycol hydrogels through the chemical and physical identity of the crosslinker, Journal of Materials Chemistry B., 2017.
- Casey, J., et al., 3D hydrogel-based microwell arrays as a tumor microenvironment model to study breast cancer growth, Biomedical Materials, 2017, 12(2):025009.
- Qayyum, A.S., et al., Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation, Biofabrication, 2017, 9(2):025019.
- Grindy, S.C., et al., Bio-inspired metal-coordinate hydrogels with programmable viscoelastic material functions controlled by longwave UV light, Soft Matter., 2017.
- Yue, X., et al., Transcriptome Profiling of 3D Co-cultured Cardiomyocytes and Endothelial Cells under Oxidative Stress Using a Photocrosslinkable Hydrogel System, Acta biomaterialia, 2017.
- Jia,W., et al., Direct 3D bioprinting of perfusable vascular constructs using a blend bioink, Biomaterials, 2016, 106, p. 58-68.
- Shah, K., et al., Development and characterization of polyethylene glycol–carbon nanotube hydrogel composite, J. Mater. Chem. B, 2015, 3, 7950-7962.
- Legros, C., Engineering of poly(2-oxazoline)s for a potential use in biomedical applications, University of Waterloo, Université de Liege and Université de Bordeaux, 2015.
- Schukur, L., et al., Directed differentiation of size-controlled embryoid bodies towards endothelial and cardiac lineages in RGD-modified poly(ethylene glycol) hydrogels. Adv Healthc Mater, 2013, 2(1): p. 195-205.
- Sugiura, S., et al., Dynamic 3D micropatterned cell co-cultures within photocurable and chemically degradable hydrogels, J Tissue Eng Regen Med, 2013.
- Pedron, S., et al., Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid, Biomaterials, 2013, 34(30), P. 7408-7417.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.