PEG products with additional MW may be made to order, please contact us for details
4arm PEG Vinylsulfone
$150.00 – $1,200.00
Description
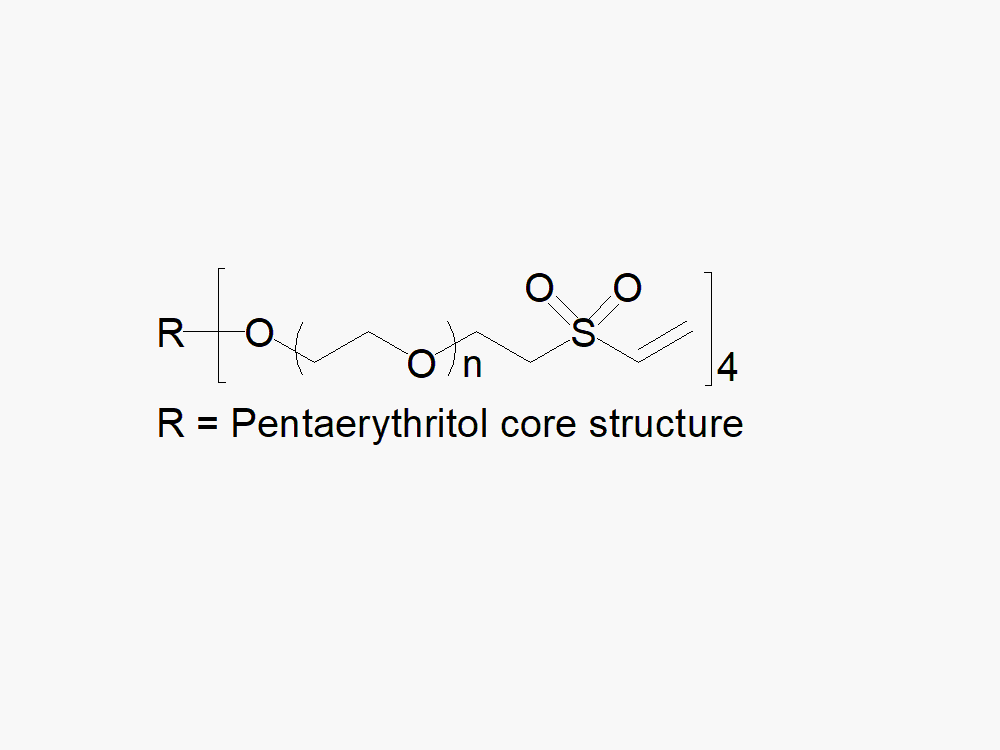
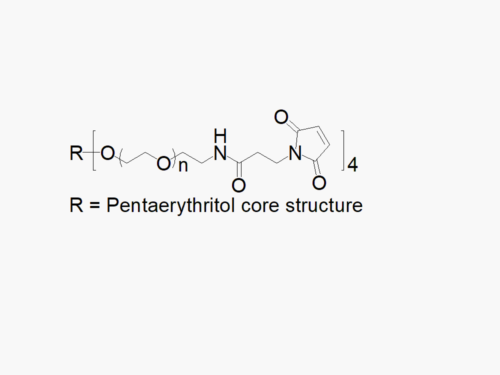
4arm PEG Vinylsulfone with superior quality specification of > 90% Substitution.
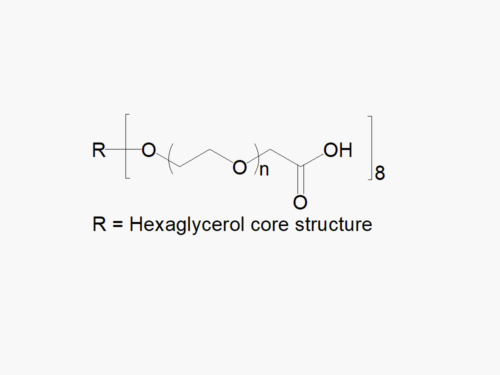
JenKem Technology’s 4arm PEG Vinylsulfone derivatives can be cross-linked into PEG hydrogels. PEG hydrogels have a variety of applications in medical devices and regenerative medicine, and are especially of interest for controlled release of drugs, for 2D and 3D cell culture, and for wound sealing and healing. JenKem Technology’s 4 arm PEGs are synthesized by ethoxylation of pentaerythritol. The number of ethylene oxide units in the PEG chain may not be equal for all arms. The total molecular weight reported for the JenKem multi-arm PEGs is the sum of the PEG molecular weights of each arm.
Multi-arm star PEG products with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Karam, J., et al., Molecular weight of hyaluronic acid crosslinked into biomaterial scaffolds affects angiogenic potential, Acta Biomaterialia, 2023.
- Lust, S.T., et al., Selectively Cross-Linked Tetra-PEG Hydrogels Provide Control over Mechanical Strength with Minimal Impact on Diffusivity. ACS Biomaterials Science & Engineering. 2021.
- Dargaville, TR, et al., Poly (2-allylamidopropyl-2-oxazoline)-Based Hydrogels: From Accelerated Gelation Kinetics to In Vivo Compatibility in a Murine Subdermal Implant Model. Biomacromolecules. 2021, 22(4):1590-9.
- Piluso, S, et al., 3D Bioprinting of molecularly engineered PEG-based hydrogels utilizing gelatin fragments. Biofabrication. 2021.
- Cha, J, et al., Cancer Cell-Sticky Hydrogels to Target the Cell Membrane of Invading Glioblastomas. ACS Applied Materials & Interfaces. 2021.
- Norman, MD, et l., Measuring the elastic modulus of soft culture surfaces and three-dimensional hydrogels using atomic force microscopy. Nature Protocols. 2021, 16(5):2418-49.
- Griffin, DR, et al., Activating an adaptive immune response from a hydrogel scaffold imparts regenerative wound healing. Nature materials. 2021, 20(4):560-9.
- Kumar, M., et al., A fully defined matrix to support a pluripotent stem cell derived multi-cell-liver steatohepatitis and fibrosis model, Biomaterials, 2021, 121006.
- Wang, J., et al., An injectable PEG hydrogel controlling neurotrophin-3 release by affinity peptides, Journal of Controlled Release, 2021, 330, P. 575-586.
- Juliar, B. A., et al., Cell-mediated matrix stiffening accompanies capillary morphogenesis in ultra-soft amorphous hydrogels, Biomaterials, 2020, V. 230.
- Rutz, A. L., et al., Employing PEG crosslinkers to optimize cell viability in gel phase bioinks and tailor post printing mechanical properties, Acta Biomaterialia, 2019.
- Van den Broeck, L., et al., Cytocompatible carbon nanotube reinforced polyethylene glycol composite hydrogels for tissue engineering, Materials Science and Engineering: C, 2019.
- Kotturi, D., et al., Evaluating hydrogels for implantable probes using SERS, InPlasmonics in Biology and Medicine XVI, 2019.
- Beamish, J.A., et al., Deciphering the relative roles of matrix metalloproteinase‐and plasmin‐mediated matrix degradation during capillary morphogenesis using engineered hydrogels, Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2019.
- Day, J.R., et al., The impact of functional groups of poly(ethylene glycol) macromers on the physical properties of photo-polymerized hydrogels and the local inflammatory response in the host, Acta Biomaterialia, 2018, 67, P. 42-52.
- Day, J.R., et al., Immunoisolating poly(ethylene glycol) based capsules support ovarian tissue survival to restore endocrine function, Journal of Biomedical Materials Research Part A., 2018.
- Schweikle, M., et al.,. Injectable synthetic hydrogel for bone regeneration: Physicochemical characterisation of a high and a low pH gelling system, Materials Science and Engineering: C, 2018, 90, pp.67-76.
- Kudva, A.K., et al., RGD‐functionalized polyethylene glycol hydrogels support proliferation and in vitro chondrogenesis of human periosteum‐derived cells, Journal of Biomedical Materials Research Part A, 2018, 106(1), p.33-42.
- Li, M., et al., Oligo (p-phenylenevinylene) Derivative-Incorporated and Enzyme-Responsive Hybrid Hydrogel for Tumor Cell-Specific Imaging and Activatable Photodynamic Therapy, ACS Biomaterials Science & Engineering, 2017.
- Kozai, T.D.Y., et al., Two-photon imaging of chronically implanted neural electrodes: Sealing methods and new insights, Journal of Neuroscience Methods, 2016, 258, P. 46-55.
- Kim, J., et al., Characterization of the crosslinking kinetics of multi-arm poly(ethylene glycol) hydrogels formed via Michael-type addition, Soft Matter, 2016.
- Darling, N.J., et al., Controlling the kinetics of thiol-maleimide Michael-type addition gelation kinetics for the generation of homogenous poly (ethylene glycol) hydrogels, Biomaterials, 2016.
- Dooling, L.J., et al., Programming Molecular Association and Viscoelastic Behavior in Protein Networks, Advanced Materials, 2016.
- Lee, W., et al., 3D patterned stem cell differentiation using thermo-responsive methylcellulose hydrogel molds, Scientific Reports, 2016, 6.
- Rosales, A.M., et al., Photoresponsive Elastic Properties of Azobenzene-Containing Poly(ethylene-glycol)-Based Hydrogels, Biomacromolecules, 2015.
- Griffin, D. R., et al., Hybrid Photopatterned Enzymatic Reaction (HyPER) for In situ Cell Manipulation, Chembiochem : a European journal of chemical biology 2014, 15(2): 233-242.
- Vigen, M., et al., Protease-Sensitive PEG Hydrogels Regulate Vascularization In Vitro and In Vivo. Macromol. Biosci., 2014, 14: 1368–1379.
- Kyburz, K.A. et al., Three-dimensional hMSC motility within peptide-functionalized PEG-based hydrogels of varying adhesivity and crosslinking density. Acta Biomaterialia, 2013, 9(5): p. 6381-6392.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.