PEG products with additional MW may be made to order, please contact us for details
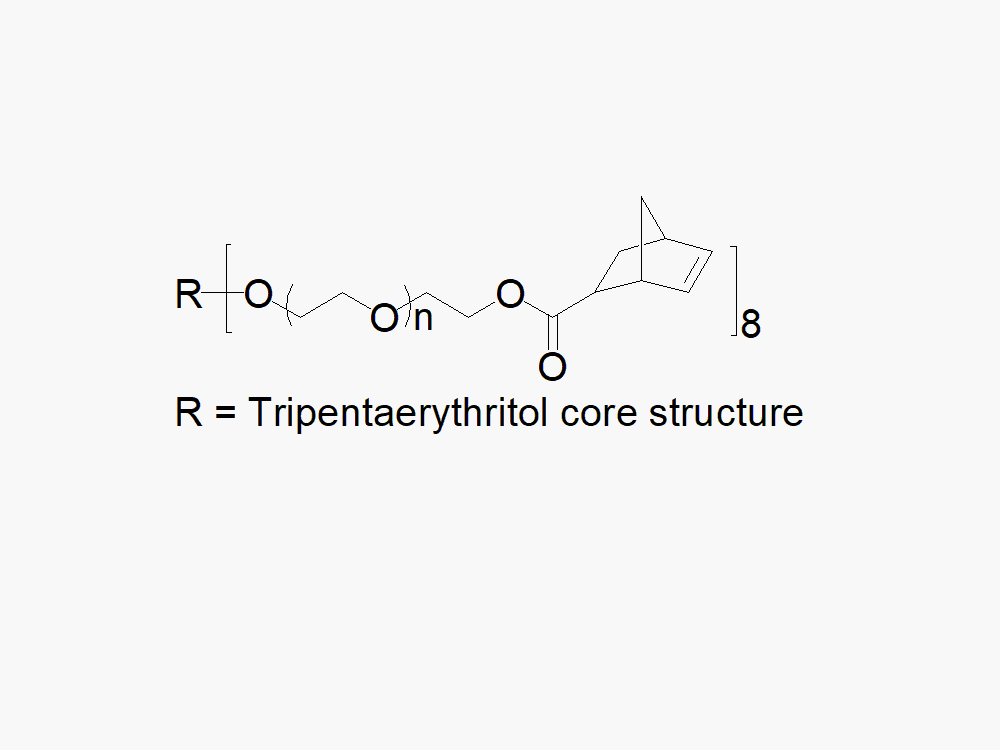
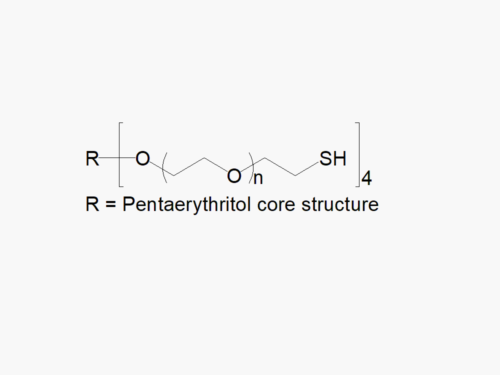
8arm PEG Norbornene (tripentaerythritol)
$150.00 – $1,200.00
Description
8arm PEG Norbornene (tripentaerythritol) reagent with high quality specification of > 85% Substitution.
JenKem Technology’s 8arm PEG Norbornene (tripentaerythritol) derivatives with hydrolytically degradable ester linkage can be crosslinked into PEG hydrogels. Norbornene PEGs are suitable for copper-free click chemistry reactions with tetrazines and for thiol-ene (thiolene) click reactions with thiols. PEG hydrogels have a variety of applications in medical devices and regenerative medicine, and are especially of interest for controlled release of drugs, for 2D and 3D cell culture, and for wound sealing and healing. Norbornene (NB) facilitates click chemistry reactions with thiols and tetrazines. JenKem Technology’s 8 arm (TP) PEGs are synthesized by ethoxylation of tripentaerythritol. 8ARM(TP)-PEG polymers with tripentaerythritol core have a higher purity as evidenced by MALDI compared with the generic 8ARM-PEG polymers with a hexaglycerin core.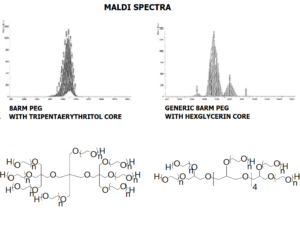
The number of ethylene oxide units in the PEG chain may not be equal for all arms. The total molecular weight reported for the JenKem multi-arm PEGs is the sum of the PEG molecular weights of each arm. Multi-arm star PEG products with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
References:
- Recktenwald, M., et al., Facile method for covalently binding peptides onto polycaprolactone films and nanofibers, Materials Letters, V. 365, 2024.
- Khang, A., et al., Estimation of aortic valve interstitial cell-induced 3D remodeling of poly(ethylene glycol) hydrogel environments using an inverse finite element approach, Acta Biomaterialia, V. 160, 2023.
- Kim MH, et al., Poly (ethylene glycol)–Norbornene as a Photoclick Bioink for Digital Light Processing 3D Bioprinting. ACS Applied Materials & Interfaces. 2023.
- Zhang, T., et al., Ellagic Acid–Cyclodextrin Inclusion Complex-Loaded Thiol–Ene Hydrogel with Antioxidant, Antibacterial, and Anti-inflammatory Properties for Wound Healing, ACS Applied Materials & Interfaces, 2023.
- Ortiz-Cárdenas, J.E., et al., Towards spatially-organized organs-on-chip: Photopatterning cell-laden thiol-ene and methacryloyl hydrogels in a microfluidic device. Organs-on-a-Chip, 2022, 100018.
- Khang, A., et al., Three-dimensional analysis of hydrogel-imbedded aortic valve interstitial cell shape and its relation to contractile behavior. Acta Biomaterialia, 2022.
- Swaminathan, G., et al., Effect of substrate stiffness on human intestinal enteroids’ infectivity by enteroaggregative Escherichia coli, Acta Biomaterialia, 2021, V. 132.
- Wilson, RL, et al, Protein-functionalized poly (ethylene glycol) hydrogels as scaffolds for monolayer organoid culture. Tissue Engineering Part C: Methods. 2021, 27(1):12-23.
- Khang, A, et al., On the Three-Dimensional Correlation Between Myofibroblast Shape and Contraction. Journal of Biomechanical Engineering. 2021, 143(9):094503.
- Grewal, MG, et al., User-defined, temporal presentation of bioactive molecules on hydrogel substrates using supramolecular coiled coil complexes. Biomaterials Science. 2021.
- Grigoryan, B, et al., Development, characterization, and applications of multi-material stereolithography bioprinting. Scientific reports. 2021, 11(1):1-3.
- Anandakrishnan, N, et al., Fast Stereolithography Printing of Large‐Scale Biocompatible Hydrogel Models. Advanced Healthcare Materials. 2021, 10(10):2002103.
- Luo, Y., et al., Light-induced dynamic RGD pattern for sequential modulation of macrophage phenotypes, Bioactive Materials, 2021, V. 6(11), P. 4065-4072.
- Sofman, M., et al., A modular polymer microbead angiogenesis scaffold to characterize the effects of adhesion ligand density on angiogenic sprouting, Biomaterials, 2021, 264, 120231
- Khang, A., et al., Quantifying heart valve interstitial cell contractile state using highly tunable poly(ethylene glycol) hydrogels, Acta Biomaterialia, 2019, 96, p. 354-367.
- Rutz, A. L., et al., Employing PEG crosslinkers to optimize cell viability in gel phase bioinks and tailor post printing mechanical properties, Acta Biomaterialia, 2019.
- Dietrich, M., et al., Guiding 3D cell migration in deformed synthetic hydrogel microstructures, Soft matter, 2018, 14(15), pp.2816-2826.
- Shukla, V., et al., Cellular Mechanics of Primary Human Cervical Fibroblasts: Influence of Progesterone and a Pro-inflammatory Cytokine, Annals of biomedical engineering, 2018, 46(1), pp.197-207.
- Dorsey, T.B., et al., Evaluation of photochemistry reaction kinetics to pattern bioactive proteins on hydrogels for biological applications, Bioactive Materials, 2017.
- Zhang, J., et al., A Genome-wide Analysis of Human Pluripotent Stem Cell-Derived Endothelial Cells in 2D or 3D Culture, Stem Cell Reports, 2017, 8:4, p. 907-918.
- Holmes, R., et al., Thiol-ene photo-click collagen-PEG hydrogels: impact of water-soluble photoinitiators on cell viability, gelation kinetics and rheological properties, Polymers, 2017, 9(6):226.
- Valdez, J., et al., On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial-stromal communication networks, Biomaterials, 2017, v. 130, p. 90-103.
- Regier, M.C., et al., The Influence of Biomaterials on Cytokine Production in 3D Cultures. Biomacromolecules. 2017, 18(3):709-18.
- Zanotelli, M.R., et al., Stable engineered vascular networks from human induced pluripotent stem cell-derived endothelial cells cultured in synthetic hydrogels, Acta biomaterialia, 2016.
- Darling, N.J., et al., Thiol-Maleimide Reaction Speed Effects on Hydrogel Homogeneity, Biomaterials, 2015.
- Le, N.N.T., et al., Hydrogel arrays formed via differential wettability patterning enable combinatorial screening of stem cell behavior, Acta Biomaterialia, 2015.
- Pellett, S., et al., Human Induced Pluripotent Stem Cell Derived Neuronal Cells Cultured on Chemically-Defined Hydrogels for Sensitive In Vitro Detection of Botulinum Neurotoxin, Scientific Reports, 2015, 5:14566.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.