PEG products with additional MW may be made to order, please contact us for details
8ARM PEG Raw Materials (hexaglycerol core)
$775.00
Description
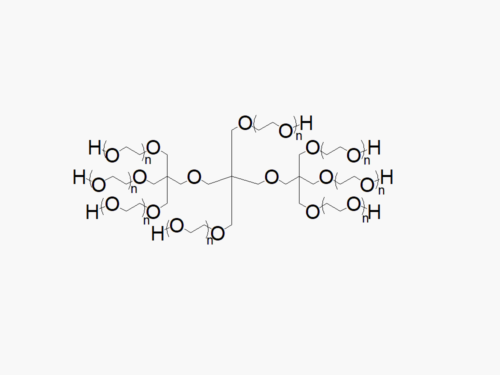
8ARM PEG Raw Materials from JenKem Technology are high quality PEGs with very low polydispersity, suitable for further derivatization into PEG derivatives and hydrogels, prepared by ethoxylation of hexaglycerol. The reported molecular weight of the multi-arm PEGs is the sum of the PEG molecular weights of each arm. The number of ethylene oxide units in the PEG chain may not be equal for all arms.
8ARM PEGs with molecular weights and branching not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
Click here to download the MSDS
| PEG PRODUCT | AVAILABLE MW | MAIN PEAK FRACTION BY GPC | POLYDISPERSITY BY GPC |
| 8ARM-PEG-OH | 10kDa, 15kDa, 20kDa, 40kDa | ≥ 95% | ≤ 1.12 |
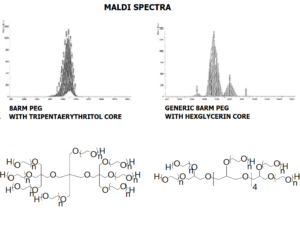
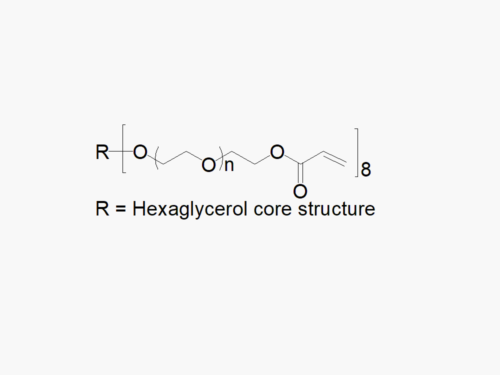
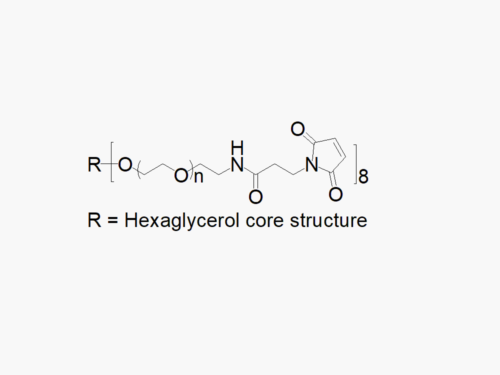
JenKem Technology also offers 8ARM(TP)-PEGs with tripentaerythritol core as an alternative to 8ARM PEGs with hexaglycerol core. 8ARM(TP)-PEGs have a higher purity as evidenced by MALDI compared with the generic 8ARM-PEGs with a hexaglycerin core.
References:
- Wang, J., et al., Quantitation of polyethylene glycol by size exclusion chromatography with charged aerosol, differential refractive index, and multi-angle light scattering detectors, Journal of Pharmaceutical and Biomedical Analysis, 238, 2024.
- Wang, R., et al., Enzymatic co-crosslinking of star-shaped poly(ethylene glycol) tyramine and hyaluronic acid tyramine conjugates provides elastic biocompatible and biodegradable hydrogels, Bioactive Materials, 20, 2023.
- Richbourg, N. R., et al., Structurally decoupled stiffness and solute transport in multi-arm poly(ethylene glycol) hydrogels, Biomaterials, 301, 2023.
- Lee, J., et al., Micromechanical property mismatch between pericellular and extracellular matrices regulates stem cell articular and hypertrophic chondrogenesis, Matter 2023, 6.2: 475-492.
- Caracena, T., et al., Transitional alveolar epithelial cells and microenvironmental stiffness synergistically drive fibroblast activation in three-dimensional hydrogel lung models. bioRxiv. 2022.
- Hewawasam, RS, et al., Chemical modification of human decellularized extracellular matrix for incorporation into phototunable hybrid-hydrogel models of tissue fibrosis. bioRxiv. 2022.
- Santoso, B., Preparation, Properties and Cell Biocompatibility of Room Temperature LCST-Hydrogels Based on Thermoresponsive PEO Stars. Gels. 2021, 7(3):84.
- Ziegler, CE, et al., In Situ Forming iEDDA Hydrogels with Tunable Gelation Time Release High-Molecular Weight Proteins in a Controlled Manner over an Extended Time. Biomacromolecules. 2021.
- Holt, SE, et al., Supramolecular Click Product Interactions Induce Dynamic Stiffening of Extracellular Matrix-Mimetic Hydrogels. Biomacromolecules. 2021.
- Zhu, D, et al., Gradient hydrogels for screening stiffness effects on patient‐derived glioblastoma xenograft cellfates in 3D. Journal of Biomedical Materials Research Part A. 2021, 109(6):1027-35.
- Wang, C, et al., Matrix Stiffness Modulates Patient-Derived Glioblastoma Cell Fates in Three-Dimensional Hydrogels. Tissue Engineering Part A. 2021, 27(5-6):390-401.
- Vedaraman, S, et al., Bicyclic RGD peptides enhance nerve growth in synthetic PEG-based Anisogels. Biomaterials Science. 2021, 9(12):4329-42.
- Li, Y., et al., Matrix metalloproteinase (MMP)-degradable tissue engineered periosteum coordinates allograft healing via early stage recruitment and support of host neurovasculature, Biomaterials, 2021, V. 268.
- Ziegler, C. E., et al., A novel anhydrous preparation of PEG hydrogels enables high drug loading with biologics for controlled release applications, European Polymer Journal, 2021, V. 147.
- Wang, C., et al., A comparative study of brain tumor cells from different age and anatomical locations using 3D biomimetic hydrogels. Acta Biomaterialia, 2020.
- Chu, S., et al., Cell encapsulation spatially alters crosslink density of poly(ethylene glycol) hydrogels formed from free-radical polymerizations, Acta Biomaterialia, 2020, 109, p. 37-50.
- Peuler, K. et al., Clickable modular polysaccharide nanoparticles for selective cell-targeting. Carbohydrate Polymers. 2020; 234:115901.
- Campbell, W. A., et al., Matrix-metalloproteinase expression and gelatinase activity in the avian retina and their influence on Müller glia proliferation, Experimental Neurology, 2019, 320.
- Graf, M., et al., Hydrogel microspheres evading alveolar macrophages for sustained pulmonary protein delivery, International Journal of Pharmaceutics, 2019, 566, p.652-661.
- Crocini, C., et al., Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness, Progress in Biophysics and Molecular Biology, 2019.
- Arkenberg, M.R., et al., Dynamic control of hydrogel crosslinking via sortase-mediated reversible transpeptidation, Acta biomaterialia, 2019, 83:83-95.
- Lin, Y., et al., Activatable cell–biomaterial interfacing with photo-caged peptides, Chemical Science, 2019, 10(4):1158-67.
- Wang, C., et al., Mimicking brain tumor-vasculature microanatomical architecture via co-culture of brain tumor and endothelial cells in 3D hydrogels, Biomaterials, 2019.
- Pinnaratip, R., et al., Effect of incorporating clustered silica nanoparticles on the performance and biocompatibility of catechol-containing PEG-based bioadhesive. Biomedical Materials, 2018, 13(2), p.025003.
- Gregoritza, M., et al., Fabrication of antibody-loaded microgels using microfluidics and thiol-ene photoclick chemistry, European Journal of Pharmaceutics and Biopharmaceutics, 2018, 127, pp.194-203.
- Buwalda, S.J., et al., Robust & thermosensitive poly (ethylene glycol)-poly (ε-caprolactone) star block copolymer hydrogels. Polymer Degradation and Stability, 2017, 137:173-83.
- Shih, H., Improving gelation efficiency and cytocompatibility of visible light polymerized thiol-norbornene hydrogels via addition of soluble tyrosine, Biomaterials Science, 2017, 5(3):589-99.
- Wang, C., et al., Effect of matrix metalloproteinase‐mediated matrix degradation on glioblastoma cell behavior in 3D PEG‐based hydrogels, Journal of Biomedical Materials Research Part A, 2017, 105(3):770-8.
- Manzoli, V., et al., Engineering human renal epithelial cells for transplantation in regenerative medicine, Medical Engineering & Physics, 2017.
- Buwalda, S. J., et al., In situ forming stereocomplexed and post-photocrosslinked acrylated star poly(ethylene glycol)-poly(lactide) hydrogels, European Polymer Journal, 2017, V. 94, P. 152-161
- Mabry, K.M., et al., Microarray analyses to quantify advantages of 2D and 3D hydrogel culture systems in maintaining the native valvular interstitial cell phenotype, Biomaterials, 2016, V. 74, P. 31-41.
- Lee, S., et al., Effects of the poly(ethylene glycol) hydrogel crosslinking mechanism on protein release, Biomater. Sci., 2016.
- Belair, D.G., et al., Human iPSC-derived endothelial cell sprouting assay in synthetic hydrogel arrays. Acta biomaterialia, 2016.
- Li, Y., et al., Gelatin Microgel Incorporated Poly (Ethylene Glycol)-based Bioadhesive with Enhanced Adhesive Property and Bioactivity. ACS applied materials & interfaces, 2016.
- Gregoritza, M., et al., Design of hydrogels for delayed antibody release utilizing hydrophobic association and Diels–Alder chemistry in tandem. Journal of Materials Chemistry B., 2016, 4(19):3398-408.
- Fasinu, P., et al., Enhancement of the Oral Bioavailability of Felodipine Employing 8-Arm-Poly (Ethylene Glycol): In Vivo, In Vitro and In Silico Evaluation. AAPS PharmSciTech, 2016, 12:1-2.
- Mabry, K.M., et al., Three-Dimensional High-Throughput Cell Encapsulation Platform to Study Changes in Cell-Matrix Interactions. ACS applied materials & interfaces, 2016.
- Gregoritza, M., et al., Polyanions effectively prevent protein conjugation and activity loss during hydrogel cross-linking, Journal of Controlled Release, 2016, 238, p. 92-102.
- Liu, H.-Y., et al., Enzyme-mediated stiffening hydrogels for probing activation of pancreatic stellate cells, Acta Biomaterialia, 2016.
- Kirchhof, S., et al., New insights into the cross-linking and degradation mechanism of Diels–Alder hydrogels, J. Mater. Chem. B, 2015, 3, 449-457.
- Hennig, R., et al., Branched Polymer–Drug Conjugates for Multivalent Blockade of Angiotensin II Receptors, Molecular Pharmaceutics, 2015, 12 (9), 3292-3302.
- Mabry, K.M., et al., Dynamic stiffening of poly(ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment, Biomaterials, 2015, 49, p: 47-56.
- Maisonneuve, B. G. C., et al., Effects of Synthetic Biomacromolecule Addition on the Flow Behavior of Concentrated Mesenchymal Cell Suspensions, Biomacromolecules, 2015, 16(1): 275-283.
- Pradal, C., et al., Hydrolytically Degradable Polyrotaxane Hydrogels for Drug and Cell Delivery Applications, Biomacromolecules, 2015, 16 (1), 389-403.
- Akalp, U., et al., Determination of the Polymer-Solvent Interaction Parameter for PEG Hydrogels in Water: Application of a Self Learning Algorithm, Polymer, 2015.
- Kirchhof, S., et al., Diels–Alder hydrogels with enhanced stability: First step toward controlled release of bevacizumab, European Journal of Pharmaceutics and Biopharmaceutics, 2015, V. 96, P. 217-225.
- Shih, H., et al., Photo-click hydrogels prepared from functionalized cyclodextrin and poly(ethylene glycol) for drug delivery and in situ cell encapsulation, Biomacromolecules, 2015.
- Wang, R., et al., Kinetically stable metal ligand charge transfer complexes as crosslinks in nanogels/hydrogels: Physical properties and cytotoxicity, Acta Biomaterialia, 2015, V. 26, P. 136-144.
- Kirchhof, S., et al., Diels–Alder Hydrogels for Controlled Antibody Release: Correlation between Mesh Size and Release Rate, Molecular Pharmaceutics, 2015, 12(9), 3358-3368.
- Van Hove, A. H., et al., Temporally Tunable, Enzymatically Responsive Delivery of Proangiogenic Peptides from Poly(ethylene glycol) Hydrogels. Advanced Healthcare Materials, 2015, 4: 2002–2011.
- Nguyen, M.K., et al., Sustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiation. Biomaterials, 2014, 35(24): p. 6278-6286.
- McKinnon, D.D., et al., Bis-Aliphatic Hydrazone-Linked Hydrogels Form Most Rapidly at Physiological pH: Identifying the Origin of Hydrogel Properties with Small Molecule Kinetic Studies,, Chemistry of Materials, 2014, 26(7): p. 2382-2387.
- Zhang, Z., et al., Synthesis of Poly(ethylene glycol)-based Hydrogels via Amine-Michael Type Addition with Tunable Stiffness and Postgelation Chemical Functionality. Chemistry of Materials, 2014, 26(12): p. 3624-3630.
- Gould, S.T., et al., The role of valvular endothelial cell paracrine signaling and matrix elasticity on valvular interstitial cell activation. Biomaterials, 2014, 35(11): p. 3596-3606.
- Lee, S., et al., The effects of varying poly(ethylene glycol) hydrogel crosslinking density and the crosslinking mechanism on protein accumulation in three-dimensional hydrogels, Acta Biomaterialia, 2014, 10(10), p: 4167–4174.
- Kirschner, C. M., et al.,Clickable, Photodegradable Hydrogels to Dynamically Modulate Valvular Interstitial Cell Phenotype, Adv Healthc Mater, 2014, 3(5): 649-657.
- Mahou, R., et al., Alginate-Poly(ethylene glycol) Hybrid Microspheres for Primary Cell Microencapsulation, Materials, 2014, 7(1), 275-286.
- Sridhar, B.V., et al., Covalently tethered TGF-β1 with encapsulated chondrocytes in a PEG hydrogel system enhances extracellular matrix production, J Biomed Mater Res Part A, 2014, 102A, 4464–4472.
- Wang, C., et al., Bioengineered 3D Brain Tumor Model To Elucidate the Effects of Matrix Stiffness on Glioblastoma Cell Behavior Using PEG-Based Hydrogels, Molecular Pharmaceutics, 2014, 11 (7), 2115-2125.
- McKinnon, D.D., et al., Measuring cellular forces using bis-aliphatic hydrazone crosslinked stress-relaxing hydrogels, Soft Matter, 2014, 10, 9230.
- Wang, R., et al., Hydrogels by supramolecular crosslinking of terpyridine end group functionalized 8-arm poly(ethylene glycol), Soft Matter, 2014, 10, 7328-7336.
- Hansen, T.D., et al., Biomaterial arrays with defined adhesion ligand densities and matrix stiffness identify distinct phenotypes for tumorigenic and non-tumorigenic human mesenchymal cell types, Biomater. Sci., 2014, 2, 745-756.
- Rudolph, T., et al., Synthesis and Solution Properties of Double Hydrophilic Poly(ethylene oxide)-block-poly(2-ethyl-2-oxazoline) (PEO-b-PEtOx) Star Block Copolymers, Polymers, 2013, 5, p: 1081-1101.
- Strehin, I., et al., Hydrogels Formed by Oxo-ester Mediated Native Chemical Ligation, Biomater Sci., 2013, 1(6), 603–613.
- Fasinu, P., et al., Flavonoids and polymer derivatives as CYP3A4 inhibitors for improved oral drug bioavailability, Journal of Pharmaceutical Sciences, 2013, 102(2): 541-555.
- Kirchhof, S., et al., Investigation of the Diels–Alder reaction as a cross-linking mechanism for degradable poly (ethylene glycol) based hydrogels, Journal of Materials Chemistry B, 2013, 1.37, 4855-4864.
- Ki, C. S., et al., Effect of 3D matrix compositions on the efficacy of EGFR inhibition in pancreatic ductal adenocarcinoma cells, Biomacromolecules, 2013, 14.9 : 3017-3026.
- Barrett, D.G., Novel Hydrogels Based on Covalent and Coordination-based cross-linking of Catechols induced by Fe3+, Polymer Preprints, 2011, 52(2), 779.
- Osman, S.K, et al., Cyclodextrin based hydrogels: Inclusion complex formation and micellization of adamantane and cholesterol grafted polymers, Polymer, 2011, 52:21, P. 4806-4812.
- Buwalda, S.J, et al., Self-Assembly and Photo-Cross-Linking of Eight-Armed PEG-PTMC Star Block Copolymers, Biomacromolecules, 2011, 12 (7), 2746-2754.
- Potta, T., et al., Design of Polyphosphazene Hydrogels with Improved Structural Properties by Use of Star-Shaped Multithiol Crosslinkers, Macromol. Biosci., 2011, 11: 689–699.
- Calucci, L., et al., Solid-State NMR Study of Stereocomplexes Formed by Enantiomeric Star-Shaped PEG–PLA Copolymers in Water, Macromolecules, 2011, 44 (18), 7288-7295.
- Maarten van Dijk, et al., Synthesis and Characterization of Enzymatically Biodegradable PEG and Peptide-Based Hydrogels Prepared by Click Chemistry, Biomacromolecules, 2010, 11(6), p: 1608–1614.
- Buwalda, S.J., et al., Influence of Amide versus Ester Linkages on the Properties of Eight-Armed PEG-PLA Star Block Copolymer Hydrogels, Biomacromolecules, 2010, 11 (1), 224-232.
- Manakker, F., et al., Supramolecular hydrogels formed by β-cyclodextrin self-association and host–guest inclusion complexes, Soft Matter, 2010, 6, 187-194.
- Manakker, F., et al., Self-Assembling Hydrogels Based on β-Cyclodextrin/Cholesterol Inclusion Complexes. Macromolecules, 2008, 41(5): p. 1766-1773.
- Manakker, F., et al., Rheological Behavior of Self-Assembling PEG-β-Cyclodextrin/PEG-Cholesterol Hydrogels, Langmuir, 2008, 24 (21), 12559-12567.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.