PEG products with additional MW may be made to order, please contact us for details
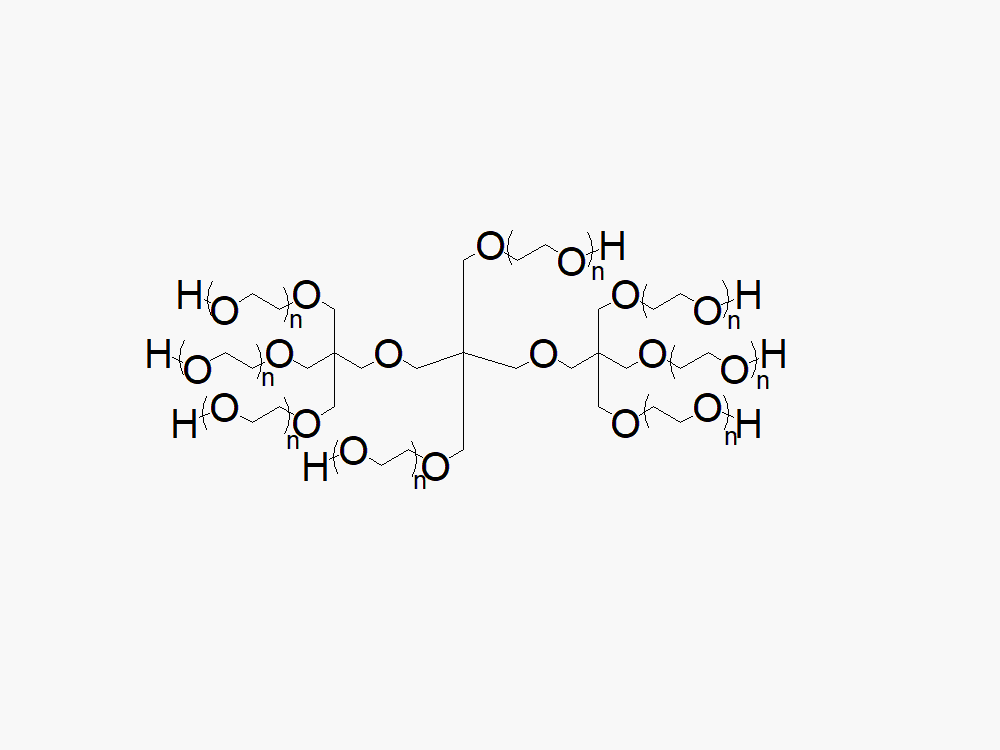
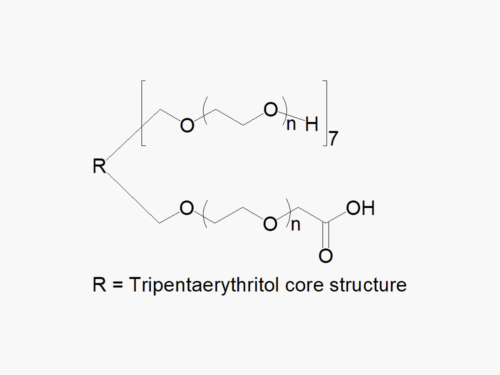
8ARM PEG Raw Materials (tripentaerythritol core)
$475.00 – $625.00
Description
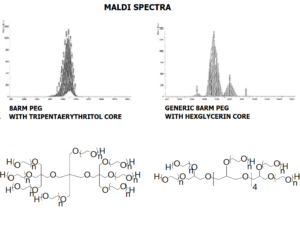
8ARM PEG (TP) Raw Materials from JenKem Technology are prepared by ethoxylation of tripentaerythritol and are suitable for further derivatization into PEG derivatives. 8ARM(TP)-PEGs with tripentaerythritol core have a higher purity as evidenced by MALDI compared with the generic 8 arm PEG Hydroxyl with hexaglycerin core.
The total molecular weight of the multi-arm PEGs is the sum of the molecular weights of all the arms. The number of ethylene oxide units in the PEG chain may not be equal for all arms.
Multi-arm PEG alcohol with molecular weights and branching not listed in our online catalog may be available by custom synthesis. Please contact us for details.
Click here to download the MSDS
| PEG PRODUCT | AVAILABLE MW | MAIN PEAK FRACTION BY GPC | POLYDISPERSITY BY GPC |
| 8ARM(TP)-PEG-OH | 10kDa, 15kDa, 20kDa, 40kDa | ≥ 95%; > 90% for MW 40,000 | ≤ 1.08 |
References:
- Wang, J., et al., Quantitation of polyethylene glycol by size exclusion chromatography with charged aerosol, differential refractive index, and multi-angle light scattering detectors, Journal of Pharmaceutical and Biomedical Analysis, 238, 2024.
- Peuler, K., et al., Clickable modular polysaccharide nanoparticles for selective cell-targeting, Carbohydrate Polymers, 2020, V. 234.
- Gangolphe, L., et al., Degradable multi (aryl azide) star copolymer as universal photo-crosslinker for elastomeric scaffolds, Materials Today Chemistry, 2019, 12:209-21.
- Saghiri, M.A., et al., Hydrogel Arrays and Choroidal Neovascularization Models for Evaluation of Angiogenic Activity of Vital Pulp Therapy Biomaterials, Journal of endodontics, 2018, 44(5), pp.773-779.
- Pushparajan, C., et al., A mechanically strengthened polyacrylamide gel matrix fully compatible with electrophoresis of proteins and nucleic acids, Electrophoresis, 2018, 39(5-6), pp.824-832.
- Dias, A.D., et al., Microcarriers with Synthetic Hydrogel Surfaces for Stem Cell Expansion, Advanced Healthcare Materials, 2017.
- Parlato, M., et al., Specific Recruitment of Circulating Angiogenic Cells Using Biomaterials as Filters, Acta Biomaterialia, 2017.
- Usprech, J., et al., Combinatorial screening of 3D biomaterial properties that promote myofibrogenesis for mesenchymal stromal cell-based heart valve tissue engineering, Acta Biomaterialia, 2017.
- Ghosh, S., et al., Strong poly(ethylene oxide) based gel adhesives via oxime cross-linking, Acta Biomaterialia, 2016, Volume 29, P.206-214.
- Lee, S., et al., Effects of the poly(ethylene glycol) hydrogel crosslinking mechanism on protein release, Biomaterials Science, 2016.
- Tong, X., et al., Hydrogels with Dual Gradients of Mechanical and Biochemical Cues for Deciphering Cell-Niche Interactions. ACS Biomaterials Science & Engineering, 2016.
- Neumann , A.J., et al., Nondestructive evaluation of a new hydrolytically degradable and photo-clickable PEG hydrogel for cartilage tissue engineering. Acta biomaterialia, 2016.
- Boere, K.W.M., et al, Thermogelling and Chemoselectively Cross-Linked Hydrogels with Controlled Mechanical Properties and Degradation Behavior, Biomacromolecules, 2015, 16 (9), 2840-2851.
- Haijiao Liu, H., et al., A microfabricated platform with hydrogel arrays for 3D mechanical stimulation of cells, Acta Biomaterialia, 2015.
- Boere, K.W.M., et al, Biofabrication of reinforced 3D-scaffolds using two-component hydrogels, J. Mater. Chem. B, 2015, 3, 9067-9078.
- Nguyen, E.H., et al., Differential effects of cell adhesion, modulus and VEGFR-2 inhibition on capillary network formation in synthetic hydrogel arrays. Biomaterials, 2014, 35(7): p. 2149-2161.
- Osman, S., et al., Self-Assembling Hydrogels Based on Β-Cyclodextrin Polymer and Poly (Ethylene Glycol) Bearing Hydrophobic Moieties for Protein Delivery, International Journal of Pharmacy and Pharmaceutical Sciences, 2014, 6(7), 591-597.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.