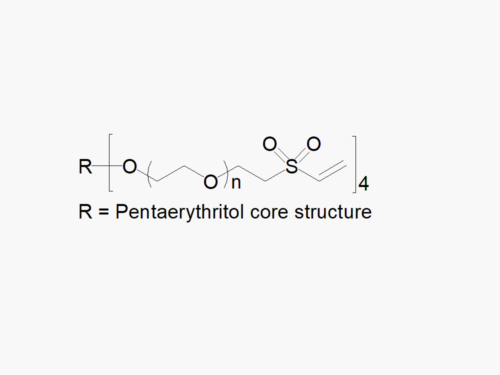
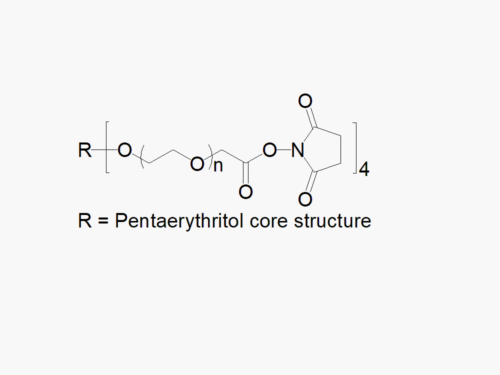
PEG products with additional MW may be made to order, please contact us for details
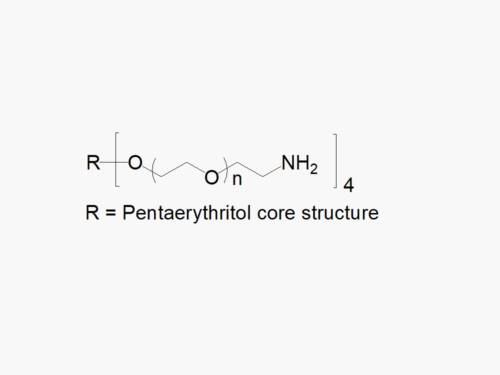
4ARM PEG Raw Materials (pentaerythritol core)
$450.00 – $625.00
Description
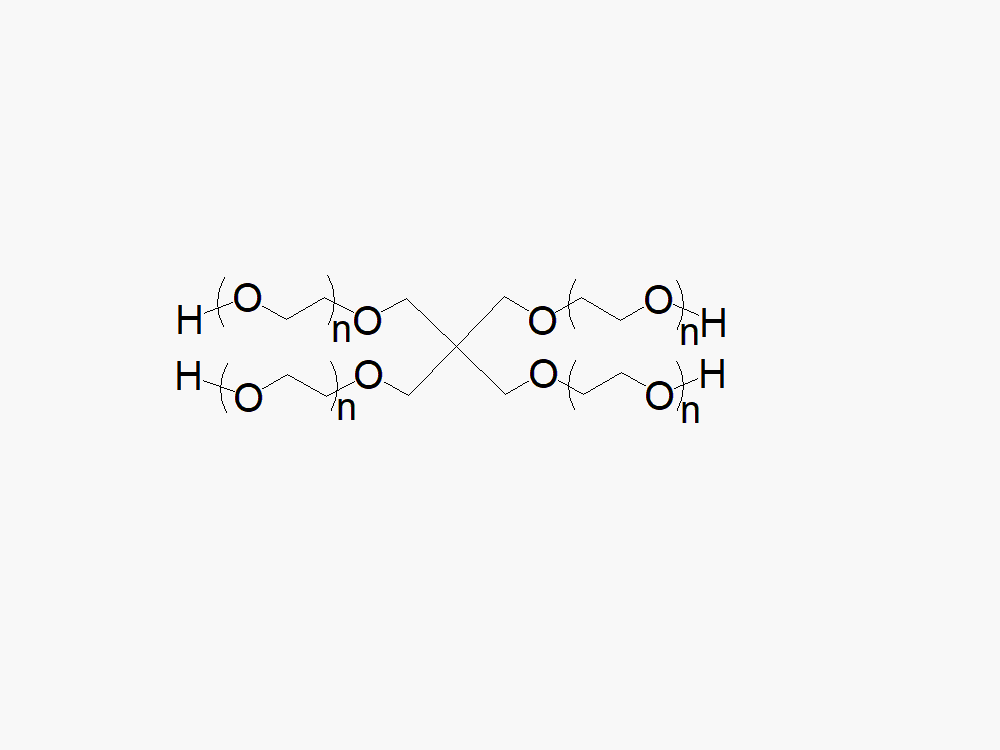
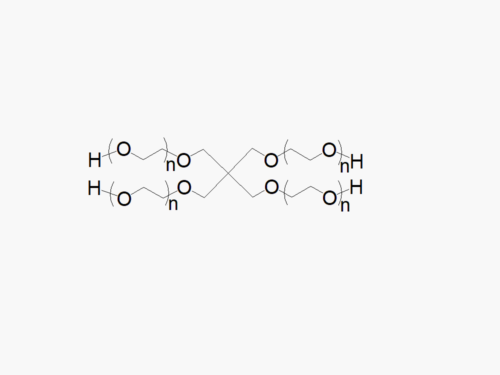
4ARM PEG Raw Materials from JenKem Technology are high quality PEGs with very low polydispersity prepared by ethoxylation of pentaerythritol, suitable for further derivatization into PEG derivatives and for hydrogel formation. The reported molecular weight of 4ARM-PEG-OH is the sum of the PEG molecular weights of each arm. The number of ethylene oxide units in the PEG chain may not be equal for all arms.
JenKem Technology also provides a 4ARM PEG GPC Calibration Standards kit (STD4ARMPEG) that includes 200mg each of 4ARM-PEG-2000, 4ARM-PEG-5000, 4ARM-PEG-10K, 4ARM-PEG-20K, and 4ARM-PEG-40K.
Multi-arm PEG raw products with molecular weights and branching not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability.
Click here to download the MSDS
| PEG PRODUCT | AVAILABLE MW | MAIN PEAK FRACTION BY GPC | POLYDISPERSITY BY GPC |
| 4ARM-PEG-OH | 2kDa, 5kDa, 15kDa, 10kDa, 20kDa | ≥ 95% | ≤ 1.05 |
References:
- Wang, J., et al., Quantitation of polyethylene glycol by size exclusion chromatography with charged aerosol, differential refractive index, and multi-angle light scattering detectors, Journal of Pharmaceutical and Biomedical Analysis, 238, 2024.
- Richbourg, N. R., et al., Structurally decoupled stiffness and solute transport in multi-arm poly(ethylene glycol) hydrogels, Biomaterials, 301, 2023.
- Riahinezhad, H., et al., Degradation of poly(vinyl sulfone carbonate) in aqueous media, Polymer Degradation and Stability, 216, 2023.
- Yan, J., et al., Combining thermosensitive physical self-assembly and covalent cycloaddition chemistry as simultaneous dual cross-linking mechanisms for the preparation of injectable hydrogels with tuneable properties, European Polymer Journal, V. 183, 2023.
- Richbourg NR, et al., Solute diffusion and partitioning in multi-arm poly (ethylene glycol) hydrogels. Journal of Materials Chemistry B. 2023; 11(2):377-88.
- Wang Y, et al., Efficacy and safety of scleral crosslinking using poly (ethylene glycol) ether tetrasuccinimidyl glutarate for form-deprivation myopia progression in rabbits. RSC Advances. 2021;11(50):31746-55.
- Santoso, B., Preparation, Properties and Cell Biocompatibility of Room Temperature LCST-Hydrogels Based on Thermoresponsive PEO Stars. Gels. 2021; 7(3):84.
- Koziol, MF, et al., Structural and Gelation Characteristics of Metallo-Supramolecular Polymer Model-Network Hydrogels Probed by Static and Dynamic Light Scattering. Macromolecules. 2021, 54(9):4375-86.
- Nicolella, P, et al., Reversible Hydrogels with Switchable Diffusive Permeability. Macromolecular Chemistry and Physics. 2021:2100076.
- Eylon, BH, et al., Injectable Drug Delivery System Based on In Situ Self‐Assembly of Liquid Star Polyethylene Glycol–Poly (lactic‐co‐glycolic acid). Advanced NanoBiomed Research. 2021, (3):2000069.
- Khodamoradi, M., et al., An electro-conductive hybrid scaffold as an artificial Bruch’s membrane, Materials Science and Engineering: C, 2021, V. 126.
- Ma, H., et al., Bioorthogonal click chemistries enable simultaneous spatial patterning of multiple proteins to probe synergistic protein effects on fibroblast function, Biomaterials, 2020, 255:120205.
- Lee, S., et al., Inflammatory responses of macrophage-like RAW264.7 cells in a 3D hydrogel matrix to ultrasonicated schizophyllan, Carbohydrate Polymers, 2020, V. 229.
- Kwak, H., et al., Colorimetric assay of tyrosinase inhibition using melanocyte laden hydrogel fabricated by digital light processing printing, Journal of Industrial and Engineering Chemistry, 2020, V. 84, P. 252-259.
- Graf, M., et al., Hydrogel microspheres evading alveolar macrophages for sustained pulmonary protein delivery, International Journal of Pharmaceutics, 2019, 566, p.652-661.
- Machado Cruz, R., et al., Impact of polyethylene glycol polymers on the physicochemical properties and mucoadhesivity of itraconazole nanoparticles, European Journal of Pharmaceutics and Biopharmaceutics, 2019, 144, p. 57-67.
- Eles, J.R., et al., Meningeal inflammatory response and fibrous tissue remodeling around intracortical implants: An in vivo two-photon imaging study, Biomaterials, 2019, 195, P. 111-12.
- Jones, C.E., et al., Stromal PTEN Regulates Extracellular Matrix Organization in the Mammary Gland, Neoplasia, 2019, 21(1):132-45.
- Xin, S., et al., Clickable PEG Hydrogel Microspheres as Building Blocks for 3D Bioprinting, Biomaterials science, 2019.
- Tae, H., et al., β-glucan hybridized poly (ethylene glycol) microgels for macrophage-targeted protein delivery, Journal of Industrial and Engineering Chemistry, 2019.
- Croitoru-Sadger, T., et al., Two-component cross-linkable gels for fabrication of solid oral dosage forms, Journal of Controlled Release, 2019.
- Kwak, H., et al., Formation of a keratin layer with silk fibroin-polyethylene glycol composite hydrogel fabricated by digital light processing 3D printing, Journal of Industrial and Engineering Chemistry, 2018.
- Shagan, A., et al., Near-Infrared Light Induced Phase Transition of Biodegradable Composites for On-Demand Healing and Drug Release, ACS applied materials & interfaces, 2018, 10(4), p. 4131-4139.
- Carthew, J., et al., Polyethylene glycol–gelatin hydrogels with tuneable stiffness prepared by horseradish peroxidase-activated tetrazine–norbornene ligation, Journal of Materials Chemistry B, 2018, 6:9, 1394-1401.
- Yue, K., et al., Visible light crosslinkable human hair keratin hydrogels, Bioengineering & translational medicine, 2018, 3(1), pp.37-48.
- Qi, D., et al., Mechanically robust cryogels with injectability and bioprinting supportability for adipose tissue engineering, Acta Biomaterialia, 2018.
- Umerska, A., at al., Freeze drying of polyelectrolyte complex nanoparticles: Effect of nanoparticle composition and cryoprotectant selection, International Journal of Pharmaceutics, 2018, V. 552 (1–2), P. 27-38.
- Kelbauskas, L., et al., A platform for high-throughput bioenergy production phenotype characterization in single cells, Scientific Reports, 2017, 7:45399.
- Truong, V.X., et al., Nonswelling Click-Cross-Linked Gelatin and PEG Hydrogels with Tunable Properties Using Pluronic Linkers, Biomacromolecules, 2017, 18(3):757-66.
- Truong, V.X., et al., Red Light Activation of Tetrazine–Norbornene Conjugation for Bioorthogonal Polymer Cross-Linking across Tissue, Chemistry of Materials, 2017, 29(8):3678-85.
- Tokuda, E.Y., et al., PEG–peptide hydrogels reveal differential effects of matrix microenvironmental cues on melanoma drug sensitivity. Integrative Biology, 2017.
- Vats, K., et al., Nanoscale physicochemical properties of chain‐and step‐growth polymerized PEG hydrogels affect cell‐material interactions. Journal of Biomedical Materials Research Part A, 2017.
- Kaya, N.U., et al., Multifunctional Dendrimer Formation Using Thiolactone Chemistry, Macromolecular Chemistry and Physics, 2017.
- Lee, S., et al., Fabrication of schizophyllan hydrogel via orthogonal thiol-ene photopolymerization, Carbohydrate Polymers, 2017.
- Liu, Y., Design Of Robust Hydrogel Based On Mussel-Inspired Chemistry, Michigan Technological University, 2017.
- Kelmansky, R., et al., In Situ Dual Cross-Linking of Neat Biogel with Controlled Mechanical and Delivery Properties, Molecular pharmaceutics, 2017, 14(10):3609-16.
- Liang, Y., et al., Liposome-crosslinked hybrid hydrogels for glutathione-triggered delivery of multiple cargo molecules, Biomacromolecules, 2016.
- Belair, D.G., et al., Regulating VEGF signaling in platelet concentrates via specific VEGF sequestering, Biomaterials Science, 2016.
- Belair, D.G., et al., Differential Regulation of Angiogenesis using Degradable VEGF-Binding Microspheres. Biomaterials, 2016, 93:27-37.
- Croitoru-Sadger, T., et al., A flexible polymersome system with tunable morphology and release profiles for efficient intracellular delivery, International Journal of Pharmaceutics, 2016, 508, 1–2, p: 34-41.
- Truong, V.X., et al., In situ-forming click-crosslinked gelatin based hydrogels for 3D culture of thymic epithelial cells, Biomaterials science, 2016.
- Ryu, S., et al., Dual mode gelation behavior of silk fibroin microgel embedded poly (ethylene glycol) hydrogel. Journal of Materials Chemistry B, 2016.
- Su, Y., et al., Mixed PEGylated surfactant modifying system decrease the accelerated blood clearance phenomenon of nanoemulsions in rats, Asian Journal of Pharmaceutical Sciences, 2016.
- Shubin, A.D., et al, Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics, Acta Biomaterialia, 2016.
- Munoz-Pinto, D.J., et al., Impact of secondary reactive species on the apparent decoupling of poly(ethylene glycol) diacrylate hydrogel average mesh size and modulus, Polymer, 2015, 77, P. 227-238.
- Shih, H., et al., Photo-click hydrogels prepared from functionalized cyclodextrin and poly(ethylene glycol) for drug delivery and in situ cell encapsulation, Biomacromolecules, 2015, 16 (7), p. 1915–1923.
- Truong, V.X., et al., Simultaneous Orthogonal Dual-Click Approach to Tough, in-Situ Forming Hydrogels for Cell Encapsulation, J. Am. Chem. Soc., 2015, 137, 1618−1622.
- Li, Q., et al., Controlling Hydrogel Mechanics via Bio-Inspired Polymer–Nanoparticle Bond Dynamics, ACS Nano, 2015.
- Kessler, M., et al., Application of Linear and Branched Poly(Ethylene Glycol)-Poly(Lactide) Block Copolymers for the Preparation of Films and Solution Electrospun Meshes. Macromol. Biosci., 2015.
- Zhou, C., et al., , Antibacterial poly(ethylene glycol) hydrogels from combined epoxy-amine and thiol-ene click reaction. J. Polym. Sci. Part A: Polym. Chem., 2015.
- Murakami, T., et al., One-pot fabrication of robust interpenetrating hydrogels via orthogonal click reactions. J. Polym. Sci. Part A: Polym. Chem., 2015.
- Shubin, A.D., et al., Development of Poly(Ethylene Glycol) Hydrogels for Salivary Gland Tissue Engineering Applications, Tissue Engineering Part A., 2015, 21(11-12): 1733-1751.
- Lin, T.-Y., et al., Designing Visible Light-Cured Thiol-Acrylate Hydrogels for Studying the HIPPO Pathway Activation in Hepatocellular Carcinoma Cells. Macromol. Biosci., 2015.
- Greene, T., et al., Modular Cross-Linking of Gelatin-Based Thiol–Norbornene Hydrogels for in Vitro 3D Culture of Hepatocellular Carcinoma Cells, ACS Biomaterials Science & Engineering, 2015, 1 (12), 1314-1323.
- Van Hove, A.H., et al., Enzymatically-responsive pro-angiogenic peptide-releasing poly(ethylene glycol) hydrogels promote vascularization in vivo, Journal of Controlled Release, 2015, V. 217, P. 191-201.
- Wu, X., et al., Nanogel-Incorporated Physical and Chemical Hybrid Gels for Highly Effective Chemo–Protein Combination Therapy. Adv. Funct. Mater., 2015, 25: 6744–6755.
- Schultz, K.M., et al., Measuring dynamic cell-material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels, Proc Natl Acad Sci U S A., 2015, 112(29):E3757-64.
- Hao, Y., et al., Visible light cured thiol-vinyl hydrogels with tunable degradation for 3D cell culture. Acta Biomaterialia, 2014, 10(1): p. 104-114.
- Daniele, M.A., et al., Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds. Biomaterials, 2014, 35(6): p. 1845-1856.
- Tokuda, E.Y., et al., Modulation of matrix elasticity with PEG hydrogels to study melanoma drug responsiveness. Biomaterials, 2014, 35(14): p. 4310-4318.
- Farooque, T.M., et al., Measuring stem cell dimensionality in tissue scaffolds. Biomaterials, 2014, 35(9): p. 2558-2567.
- Lin, T.-Y., C.S. Ki, and C.-C. Lin, Manipulating hepatocellular carcinoma cell fate in orthogonally cross-linked hydrogels. Biomaterials, 2014, 35(25): p. 6898-6906.
- Jeong, C.G., et al., Screening of hyaluronic acid–poly(ethylene glycol) composite hydrogels to support intervertebral disc cell biosynthesis using artificial neural network analysis. Acta Biomaterialia, 2014, 10(8): p. 3421-3430.
- Xu, J., E. Feng, and J. Song, Bioorthogonally Cross-Linked Hydrogel Network with Precisely Controlled Disintegration Time over a Broad Range. Journal of the American Chemical Society, 2014, 136(11): p. 4105-4108.
- Azagarsamy, M.A., et al., Coumarin-Based Photodegradable Hydrogel: Design, Synthesis, Gelation, and Degradation Kinetics. ACS Macro Letters, 2014, 3(6): p. 515-519.
- Van Hove, A.H., et al.,, Development and in vitro assessment of enzymatically-responsive poly(ethylene glycol) hydrogels for the delivery of therapeutic peptides, Biomaterials, 2014, 35(36), p: 9719-9730.
- Hammer, N., et al., Cleavable carbamate linkers for controlled protein delivery from hydrogels, Journal of Controlled Release, 2014, 183, P. 67-76.
- Fraser, A. K., et al., PEG-Based Microgels Formed by Visible-Light-Mediated Thiol-Ene Photo-Click Reactions. Macromol. Chem. Phys., 2014, 215: 507–515.
- Kharkar, P.M., et al., Dually degradable click hydrogels for controlled degradation and protein release, J. Mater. Chem. B, 2014, 2, 5511-5521.
- Belair, D.G., et al., Serum-Dependence of Affinity-Mediated VEGF Release from Biomimetic Microspheres, Biomacromolecules, 2014, 15 (6), 2038-2048.
- Munoz, Z., et al., Gelatin hydrogels formed by orthogonal thiol–norbornene photochemistry for cell encapsulation, Biomater. Sci., 2014, 2, 1063-1072.
- Ki, C.S., et al., Facile preparation of photodegradable hydrogels by photopolymerization, Polymer (Guildf), 2013, 54(8): 2115–2122.
- Raza, A., et al., The Influence of Matrix Degradation and Functionality on Cell Survival and Morphogenesis in PEG-Based Hydrogels, Macromolecular Bioscience, 2013, 13(8): 1048-1058.
- Barrett, D. G., et al., Mechanically Robust, Negative-Swelling, Mussel-Inspired Tissue Adhesives, Adv Healthc Mater, 2013, 2(5): 745-755.
- Shih, H., et al., Visible-Light-Mediated Thiol-Ene Hydrogelation Using Eosin-Y as the Only Photoinitiator, Macromolecular Rapid Communications, 2013, 34(3): 269-273.
- Fasinu, P., et al., Flavonoids and polymer derivatives as CYP3A4 inhibitors for improved oral drug bioavailability, Journal of Pharmaceutical Sciences, 2013, 102(2): 541-555.
- McGann, C. L., et al., Resilin-Based Hybrid Hydrogels for Cardiovascular Tissue Engineering, Macromolecules, 2013, 214(2): 203-213.
- Schultz, K. M., et al., Monitoring degradation of matrix metalloproteinases-cleavable PEG hydrogels via multiple particle tracking microrheology, Soft Matter, 2013, 1570-1579.
- Kirchhof, S., et al., Investigation of the Diels–Alder reaction as a cross-linking mechanism for degradable poly (ethylene glycol) based hydrogels, Journal of Materials Chemistry B, 2013, 1.37, : 4855-4864.
- Baldwin AD, et al., Reversible maleimide–thiol adducts yield glutathione-sensitive poly (ethylene glycol)–heparin hydrogels. Polymer chemistry, 2013, 4(1):133-43.
- Missirlis, D., et al., Combined effects of PEG hydrogel elasticity and cell-adhesive coating on fibroblast adhesion and persistent migration. Biomacromolecules, 2013, 15.1 : 195-205.
- Shih, H., et al., Interfacial thiol-ene photoclick reactions for forming multilayer hydrogels. ACS applied materials & interfaces, 2013, 5(5):1673-80.
- Raza, A., et al., The influence of matrix properties on growth and morphogenesis of human pancreatic ductal epithelial cells in 3D. Biomaterials, 2013, 34(21):5117-27.
- Belair, D.G., et al., Specific VEGF sequestering to biomaterials: Influence of serum stability. Acta biomaterialia, 2013, 9(11):8823-31.
- Kyburz, K.A., et al., Three-dimensional hMSC motility within peptide-functionalized PEG-based hydrogels of varying adhesivity and crosslinking density. Acta biomaterialia, 2013, 9(5):6381-92.
- Leight, J.L., et al., Direct measurement of matrix metalloproteinase activity in 3D cellular microenvironments using a fluorogenic peptide substrate. Biomaterials, 2013, 34(30):7344-52.
- Baldwin, A. D., et al., In situ crosslinkable heparin-containing poly(ethylene glycol) hydrogels for sustained anticoagulant release, J Biomed Mater Res, 2012, A 100(8): 2106-2118.
- Browning, M. B., et al., Compositional control of poly(ethylene glycol) hydrogel modulus independent of mesh size, Journal of Biomedical Materials Research, 2011, 98A(2): 268-273.
- Xu, J., et al., Cytocompatible poly(ethylene glycol)-co-polycarbonate hydrogels cross-linked by copper-free, strain-promoted click chemistry, Chem Asian J, 2011, 6(10): 2730-2737.
- Anderson, S.B. et al., The performance of human mesenchymal stem cells encapsulated in cell-degradable polymer-peptide hydrogels, Biomaterials, 2011, 32:14, P. 3564-3574.
- Lin, C.C., et al., PEG hydrogels formed by thiol-ene photo-click chemistry and their effect on the formation and recovery of insulin-secreting cell spheroids, Biomaterials, 2011, 32:36, P. 9685-9695.
- Fairbanks, B.D., et al., Photodegradable, Photoadaptable Hydrogels via Radical-Mediated Disulfide Fragmentation Reaction, Macromolecules, 2011, 44 (8), 2444-2450.
- Pek, Y.S., et al., Characterization of early extracellular matrix secretions in a three-dimensional thixotropic cell culture system, Acta Biomaterialia, 2011, 7:11, P. 3981-3987.
- Xu, J., et al., Cytocompatible Poly(ethylene glycol)-co-polycarbonate Hydrogels Crosslinked by Copper-free, Strain-promoted “Click” Chemistry, Chemistry, an Asian journal, 2011, 6(10):2730-2737.
- Maarten van Dijk, et al., Synthesis and Characterization of Enzymatically Biodegradable PEG and Peptide-Based Hydrogels Prepared by Click Chemistry, Biomacromolecules, 2010, 11(6) p: 1608–1614.
- Murphy, J.L., et al., Adhesive Performance of Biomimetic Adhesive-Coated Biologic Scaffolds, Biomacromolecules, 2010, 11(11), p. 2976–2984.
- Jin, R., et al., Synthesis and characterization of hyaluronic acid–poly(ethylene glycol) hydrogels via Michael addition: An injectable biomaterial for cartilage repair, Acta Biomaterialia, 2010, 6:6, P. 1968-1977.
- F. Tewes, et al., Development and characterisation of soluble polymeric particles for pulmonary peptide delivery, European Journal of Pharmaceutical Sciences, 2010, 41:2, P. 337-352.
- Jin, R., Injectable Hydrogels for Cartilage Tissue Engineering, University of Twente, 2009.
- Fechler, N., et al., Thermogelation of PEG-Based Macromolecules of Controlled Architecture, Macromolecules, 2009, 42 (1), 33-36.
- Aimetti, A.A., et al., Poly(ethylene glycol) hydrogels formed by thiol-ene photopolymerization for enzyme-responsive protein delivery, Biomaterials, 2009, 30:30, P. 6048-6054.
- Nezha Badi, N., et al., PEG-based thermogels: Applicability in physiological media, Journal of Controlled Release, 2009, 140:3, P. 224-229.
- Manakker, F., et al., Rheological Behavior of Self-Assembling PEG-β-Cyclodextrin/PEG-Cholesterol Hydrogels, Langmuir, 2008, 24 (21), 12559-12567.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.