PEG products with additional MW may be made to order, please contact us for details
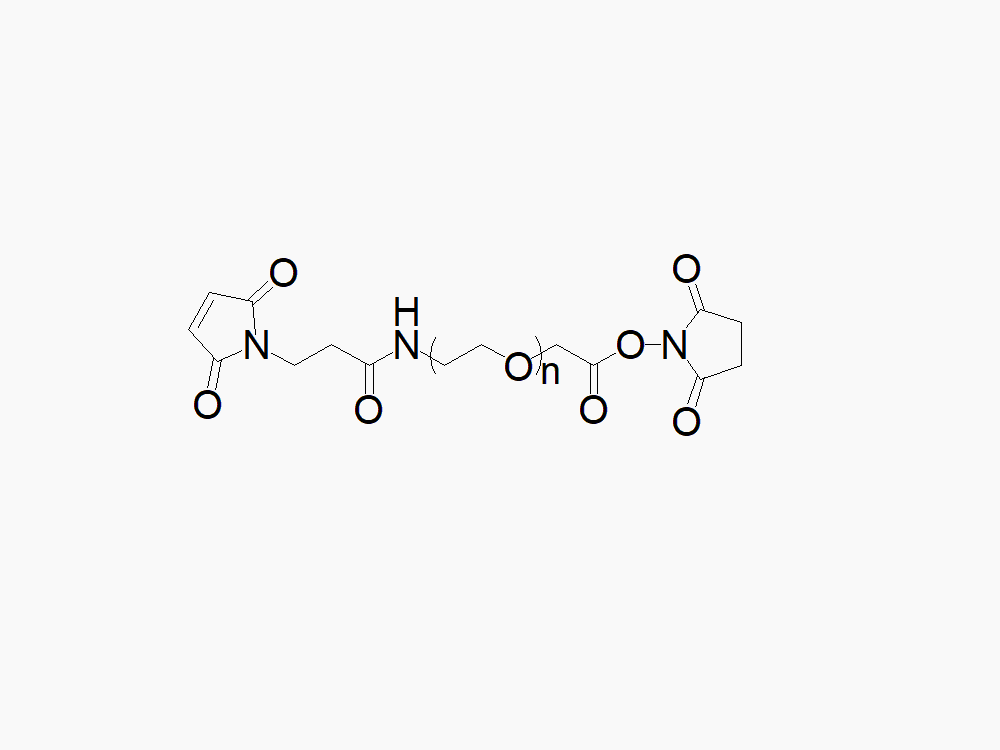
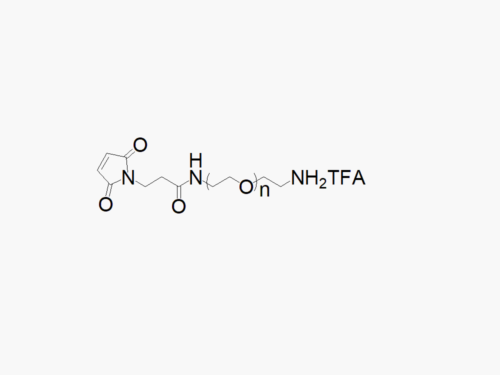
Maleimide PEG Succinimidyl Carboxymethyl Ester
$425.00 – $1,800.00
Description
High quality Maleimide PEG Succinimidyl Carboxymethyl Ester (MAL-PEG-SCM) with a standard quality specification of >90% Substitution.
Heterobifunctional Maleimide PEG Succinimidyl Carboxymethyl Ester products from JenKem Technology are generally employed as crosslinking agents or as spacers between two different chemical entities. The PEG moiety in the heterofunctional PEG derivatives provides water solubility, biocompatibility, and flexibility. Applications are especially geared towards the development of antibody drug conjugates (ADC’s).
Heterobifunctional PEGylation reagents with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability of custom PEGs.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
Note: Starting July 2016, Maleimide PEG Succinimidyl Carboxymethyl Ester is the new name of the product Maleimide PEG NHS (MW 2000 (MAL-PEG2000-NHS), MW 3500 (MAL-PEG3500-NHS), MW 5000 (MAL-PEG5000-NHS) and MW 7500 (MAL-PEG7500-NHS)).
References:
- Chen, H, et al., Biomimetic Lipopolysaccharide‐Free Bacterial Outer Membrane‐Functionalized Nanoparticles for Brain‐Targeted Drug Delivery. Advanced Science. 2022.
- Zhang, S., et al., Brain-targeting, acid-responsive antioxidant nanoparticles for stroke treatment and drug delivery. Bioactive materials, 2022, 16.
- Cheng, Y., et al, Light-switchable diphtherin transgene system combined with losartan for triple negtative breast cancer therapy based on nano drug delivery system. International Journal of Pharmaceutics, 2022, p.121613.
- Wang, P., et al., Precise gene delivery systems with detachable albumin shell remodeling dysfunctional microglia by TREM2 for treatment of Alzheimer’s disease, Biomaterials, 2022, V. 281
- Fonseca, DR, et al., Grafting MSI-78A onto chitosan microspheres enhances its antimicrobial activity. Acta Biomaterialia. 2022, 137:186-98
- Fotouhi, P., et al., Surface modified and rituximab functionalized PAMAM G4 nanoparticle for targeted imatinib delivery to leukemia cells: In vitro studies, Process Biochemistry, 2021, V. 111 (1).
- Ju, X., et al., Prodrug Delivery Using Dual-Targeting Nanoparticles To Treat Breast Cancer Brain Metastases. Molecular Pharmaceutics. 2021.
- Ren, M., et al., An oligopeptide/aptamer-conjugated dendrimer-based nanocarrier for dual-targeting delivery to bone. Journal of Materials Chemistry B. 2021, 9(12):2831-44.
- Ju, X, et al., Overcoming Mfsd2a‐Mediated Low Transcytosis to Boost Nanoparticle Delivery to Brain for Chemotherapy of Brain Metastases. Advanced Healthcare Materials. 2021, 10(9):2001997.
- Ngamcherdtrakul, W, et al., In Situ Tumor Vaccination with Nanoparticle Co‐Delivering CpG and STAT3 siRNA to Effectively Induce Whole‐Body Antitumor Immune Response. Advanced Materials. 2021, 12:2100628.
- Li, Q, et al., PEG Linker Improves Antitumor Efficacy and Safety of Affibody-Based Drug Conjugates. International journal of molecular sciences. 2021, 22(4):1540.
- Steinbauer, P., et al., Biomimetic adhesion motifs based on RAFT polymers with phosphonate groups, European Polymer Journal, 2021, V. 143.
- JNi, J., et al., PSMA-targeted nanoparticles for specific penetration of blood-brain tumor barrier and combined therapy of brain metastases, Journal of Controlled Release, 2021, V. 329, P. 934-947.
- Khan, N.U., et al., Escape from abluminal LRP1-mediated clearance for boosted nanoparticle brain delivery and brain metastasis treatment, Acta Pharmaceutica Sinica B, 2020.
- Ferreira, N.N., et al., Validation of an innovative analytical method for simultaneous quantification of alpha-cyano-4-hydroxycinnamic acid and the monoclonal antibody cetuximab using HPLC from PLGA-based nanoparticles, Journal of Pharmaceutical and Biomedical Analysis, 2020, 190, 113540.
- Jiang, H., et al., Multimodal theranostics augmented by transmembrane polymer-sealed nano-enzymatic porous MoS2 nanoflowers, International Journal of Pharmaceutics, 2020, 586, 119606.
- Hou, X., et al., A combination of LightOn gene expression system and tumor microenvironment-responsive nanoparticle delivery system for targeted breast cancer therapy, Acta Pharmaceutica Sinica B., 2020.
- Xu, Y., et al., Cell-Penetrating Peptide Enhanced Insulin Buccal Absorption, International Journal of Pharmaceutics, 2020:119469.
- Keuth, J., et al., Reversion of arterial calcification by elastin-targeted DTPA-HSA nanoparticles, European Journal of Pharmaceutics and Biopharmaceutics, 2020, V. 150, P. 108-119.
- He, M., et al., Spatiotemporally controllable diphtheria toxin expression using a light-switchable transgene system combining multifunctional nanoparticle delivery system for targeted melanoma therapy, Journal of Controlled Release, 2020, V. 319, P. 1-14.
- Wan, W., et al., Doxorubicin and siRNA-PD-L1 co-delivery with T7 modified ROS-sensitive nanoparticles for tumor chemoimmunotherapy, International Journal of Pharmaceutics, 2019, 566, p. 731-744.
- Säälik, P., et al., Peptide-guided nanoparticles for glioblastoma targeting, Journal of Controlled Release, 2019, 308, p. 109-118.
- Xiang, Y., et al., tLyp-1-conjugated GSH-sensitive biodegradable micelles mediate enhanced pUNO1-hTRAILa/curcumin co-delivery to gliomas, Chemical Engineering Journal, 2019, V. 374, P. 392-404.
- Guo, Q., et al., LRP1-upregulated nanoparticles for efficiently conquering the blood-brain barrier and targetedly suppressing multifocal and infiltrative brain metastases, Journal of Controlled Release, 2019, v. 303, 2019, p. 117-129.
- Liu, L., et al., Photoacoustic Therapy for Precise Eradication of Glioblastoma with a Tumor Site Blood–Brain Barrier Permeability Upregulating Nanoparticle, Advanced Functional Materials, 2019, 1808601.
- Song, Z., et al., Phenylboronic acid-functionalized polyamidoamine-mediated miR-34a delivery for the treatment of gastric cancer, Biomaterials Science, 2019.
- Feng, J., et al., Dendritic Polylysine Based ανβ3 Integrin Targeted Probe for Near-infrared Fluorescent Imaging of Glioma, Colloids and Surfaces B: Biointerfaces, 2019.
- Ngamcherdtrakul, W., et al., Lanthanide-Loaded Nanoparticles as Potential Fluorescent and Mass Probes for High-Content Protein Analysis, Bioengineering, 2019, 6(1):23.
- Saqafi, B., et al., Polyethyleneimine-polyethylene glycol copolymer targeted by anti-HER2 nanobody for specific delivery of transcriptionally targeted tBid containing construct, Artificial cells, nanomedicine, and biotechnology, 2019.
- Narmani, A., et al., Synthesis and evaluation of polyethylene glycol-and folic acid-conjugated polyamidoamine G4 dendrimer as nanocarrier, Journal of Drug Delivery Science and Technology, 2019.
- Han, S., et al., A novel synergetic targeting strategy for glioma therapy employing borneol combination with angiopep-2-modified, DOX-loaded PAMAM dendrimer, Journal of drug targeting, 2018, 26(1), pp.86-94.
- Qi, H., et al., Exosomes separated based on the “STOP” criteria for tumor-targeted drug delivery, Journal of Materials Chemistry B, 2018, 6(18), pp.2758-2768.
- Li, H., et al., Dendron‐Grafted Polylysine‐Based Dual‐Modal Nanoprobe for Ultra‐Early Diagnosis of Pancreatic Precancerosis via Targeting a Urokinase‐Type Plasminogen Activator Receptor, Advanced healthcare materials, 2018, 7(5), p.1700912.
- Hong, S.S., et al., Follicle-stimulating hormone peptide-conjugated nanoparticles for targeted shRNA delivery lead to effective gro-α silencing and antitumor activity against ovarian cancer, Drug delivery, 2018, 25(1), pp.576-584.
- Zhang, M.X., et al., Transcriptional control of the MUC16 promoter facilitates follicle-stimulating hormone peptide-conjugated shRNA nanoparticle-mediated inhibition of ovarian carcinoma in vivo, Drug delivery, 2018, 25(1), pp.797-806.
- Hu, Q., et al., Chondrocyte affinity peptide modified PAMAM conjugate as a nanoplatform for targeting and retention in cartilage, Nanomedicine, 2018, 13(7), pp.749-767.
- Singh, A.A.G.C., et al., Tissue-engineered Neo-Urinary Conduit from Decellularized Trachea, Tissue Engineering, 2018.
- Zhang, L., et al., In vivo tumor active cancer targeting and CT-fluorescence dual-modal imaging with nanoprobe based on gold nanorods and InP/ZnS quantum dots, Journal of Materials Chemistry B, 2018.
- Li, M., Single-Molecule Recognition and Force Measurements by AFM, Investigations of Cellular and Molecular Biophysical Properties by Atomic Force Microscopy Nanorobotics, 2018, pp. 49-64.
- Zhou, F., et al., Electrospun membranes of PELCL/PCL-REDV loading with miRNA-126 for enhancement of vascular endothelial cell adhesion and proliferation, Materials Science and Engineering: C, 2018, V. 85, P. 37-46.
- Narmani, A., et al., Targeting delivery of oxaliplatin with smart PEG-modified PAMAM G4 to colorectal cell line: In vitro studies, Process Biochemistry, 2018.
- Ruan, C., et al., Substance P-modified human serum albumin nanoparticles loaded with paclitaxel for targeted therapy of glioma, Acta Pharmaceutica Sinica B, 2018, V. 8, Issue 1, P. 85-96.
- Zhang, Y., et al., Endogenous albumin-mediated delivery of redox-responsive paclitaxel-loaded micelles for targeted cancer therapy, Biomaterials, 2018, V. 183, P. 243-257.
- Rajkumar, S., et al., Multi-functional nanocarriers based on iron oxide nanoparticles conjugated with doxorubicin, poly(ethylene glycol) and folic acid as theranostics for cancer therapy, Colloids and Surfaces B: Biointerfaces, 2018, V. 170, P. 529-537.
- Rajkumar, S., et al., Multi-functional core-shell Fe3O4@Au nanoparticles for cancer diagnosis and therapy, Colloids and Surfaces B: Biointerfaces, 2019, Vol. 174, P. 252-259.
- Han, X., et al., Free paclitaxel-loaded E-selectin binding peptide modified micelle self-assembled from hyaluronic acid-paclitaxel conjugate inhibit breast cancer metastasis in a murine model, International Journal of Pharmaceutics, 2017.
- Wang, S., et al., Augmented glioma-targeted theranostics using multifunctional polymer-coated carbon nanodots, Biomaterials, 2017.
- Wu, Z., et al., RGD/CTX-Conjugated Multifunctional Eu-Gd2O3 NRs for Targeting Detection and Inhibition of Early Tumour, Journal of Materials Chemistry B, 2017.
- Jiang, G., et al., Formulation of temozolomide-loaded nanoparticles and their targeting potential to melanoma cells, Oncology reports, 2017, 37(2):995-1001.
- Chang, X., et al., Conjugation of PEG-hexadecane markedly increases the immunogenicity of pneumococcal polysaccharide conjugate vaccine, Vaccine, 2017, 35(13):1698-704.
- Mesken, J., et al., Modifying plasmid-loaded HSA-nanoparticles with cell penetrating peptides–Cellular uptake and enhanced gene delivery, International journal of pharmaceutics, 2017, 522(1):198-209.
- Liu, C., et al., iRGD-mediated core-shell nanoparticles loading carmustine and O6-benzylguanine for glioma therapy, Journal of Drug Targeting, 2017, 25(3):235-46.
- Lopes, C.D.F., et al., BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury, Biomaterials, 2017, V. 121, P. 83-96
- Nascimento, A.V., et al., Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNAdelivered systemically using EGFR-targeted chitosan nanoparticles, Acta Biomaterialia, 2017, V. 47, P. 71-80.
- Lv, J., et al., Star-shaped copolymer grafted PEI and REDV as a gene carrier to improve migration of endothelial cells. Biomaterials Science, 2017.
- Chen, H., et al., Dual aptamer modified dendrigraft poly-L-lysine nanoparticles for overcoming multi-drug resistance through mitochondrial targeting. Journal of Materials Chemistry B., 2017.
- Dadras, P., et al., Formulation and evaluation of targeted nanoparticles for breast cancer theranostic system. European Journal of Pharmaceutical Sciences, 2017, 97:47-54.
- Ji, Y., et al., A Novel Pseudo-Protein-Based Biodegradable Nanomicellar Platform for the Delivery of Anticancer Drugs, Small, 2017, 13, 1601491.
- Sharma, S., et al., Tumor-penetrating nanosystem strongly suppresses breast tumor growth. Nano Letters, 2017, 17(3):1356-64.
- Han, S., et al., A Novel Synergetic Targeting Strategy for Glioma Therapy Employing Borneol Combination with Angiopep-2-modified, DOX-loaded PAMAM Dendrimer, Journal of Drug Targeting, 2017, 1-20.
- Hunt, H., et al., Targeting of p32 in peritoneal carcinomatosis with intraperitoneal linTT1 peptide-guided pro-apoptotic nanoparticles, Journal of Controlled Release, 2017.
- Zhang, C., et al., Development of long-acting ciliary neurotrophic factor by site-specific conjugation with different-sized polyethylene glycols and transferrin, International Journal of Pharmaceutics, 2017.
- Kiel, S., et al., Engineered Doxorubicin Delivery System Using Proteinoid-Poly (L-Lactic Acid) Polymeric Nanoparticles of Narrow Size Distribution and High Molecular Weight for Cancer Treatment, International Journal of Nanotechnology & Nanomedicine, 2017, V. 2:1.
- Hunt, H., et al., Targeting of p32 in peritoneal carcinomatosis with intraperitoneal linTT1 peptide-guided pro-apoptotic nanoparticles, Journal of Controlled Release, 2017, 260, P. 142-153.
- Yuan, Z., et al., Multifunctional nanoparticles co-delivering EZH2 siRNA and etoposide for synergistic therapy of orthotopic non-small-cell lung tumor, Journal of Controlled Release, 2017, v. 268, P. 198-211.
- Sharma, S., et al., Vascular changes in tumors resistant to a vascular disrupting nanoparticle treatment, Journal of Controlled Release, 2017, v. 268, p. 49-56.
- Narmani, A., et al., Imaging, biodistribution and in vitro study of smart 99mTc-PAMAM G4 dendrimer as novel nano-complex, Colloids and Surfaces B: Biointerfaces, 2017, V. 159, P. 232-240.
- Mesken, J., et al., EB1 modified HSA nanoparticles as non-viral delivery vectors-Influence of peptide concentration on cell uptake, Materials Today: Proceedings, 2017, 4:S174-9.
- Liu, Y., et al., Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer’s disease mice, Biomaterials, 2016, Volume 80, P. 33-45.
- Xu, Z., et al., A poly(amidoamine) dendrimer-based nanocarrier conjugated with Angiopep-2 for dual-targeting function in treating glioma cells, Polym. Chem., 2016, 7, 715-721.
- Gao, S., et al., Plasmid pORF-hTRAIL targeting to glioma using transferrin-modified polyamidoamine dendrimer. Drug Design, Development and Therapy, 2016, 10:1-11.
- Liu, X., et al., Tumor-Targeted Multimodal Optical Imaging with Versatile Cadmium-Free Quantum Dots, Adv. Funct. Mater., 2016, 26: 267–276.
- Li, J., et al., Synthesis of a bi-functional dendrimer-based nanovehicle co-modified with RGDyC and TAT peptides for neovascular targeting and penetration, International Journal of Pharmaceutics, 2016, V. 501:1–2, P. 112-123.
- Zhao, L., et al., Computational design of peptide-Au cluster probe for sensitive detection of αIIbβ3 integrin, Nanoscale, 2016, 8, 4203-4208.
- Nascimento, A.V., et al., Biodistribution and pharmacokinetics of Mad2 siRNA-loaded EGFR-targeted chitosan nanoparticles in cisplatin sensitive and resistant lung cancer models. Nanomedicine, 2016, 11(7), pp.767-781.
- Kim, J.B., et al., Intravascular optical imaging of high-risk plaques in vivo by targeting macrophage mannose receptors. Scientific reports, 6, 2016.
- Golan, M., et al., Inhibition of Gene Expression and Cancer Cell Migration by CD44v3/6-Targeted Polyion Complexes, Bioconjugate Chemistry, 2016.
- Yang, J., et al., REDV–polyethyleneimine complexes for selectively enhancing gene delivery in endothelial cells. Journal of Materials Chemistry B, 2016, 4(19):3365-76.
- Luo, B., et al., Conjugation Magnetic PAEEP-PLLA Nanoparticles with Lactoferrin as a Specific Targeting MRI Contrast Agent for Detection of Brain Glioma in Rats. Nanoscale research letters, 2016, 11(1):1-1.
- Han, L., et al., Targeted drug delivery to ischemic stroke via chlorotoxin-anchored, lexiscan-loaded nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 2016.
- Liu, J., et al., Single-molecule force spectroscopy study of the effect of cigarette carcinogens on thrombomodulin–thrombin interaction. Science Bulletin, 2016, 8.
- Li, M., et al., Rapid recognition and functional analysis of membrane proteins on human cancer cells using atomic force microscopy, Journal of Immunological Methods, 2016.
- Li, F., et al., Non-invasively differentiating extent of liver fibrosis by visualizing hepatic integrin αvβ3 expression with an MRI modality in mice, Biomaterials, 2016, 102:162-74.
- Gao, X., et al., Nanoagonist-mediated endothelial tight junction opening: A strategy for safely increasing brain drug delivery in mice, Journal of Cerebral Blood Flow & Metabolism, 2016.
- Lopes, C.D., et al., In vivo targeted gene delivery to peripheral neurons mediated by neurotropic poly (ethylene imine)-based nanoparticles, International Journal of Nanomedicine, 2016, 11:2675.
- Wu, D., et al., Phenylboronic acid-functionalized polyamidoamine-mediated Bcl-2 siRNA delivery for inhibiting the cell proliferation, Colloids and Surfaces B: Biointerfaces, 2016, 146:318-25.
- Chen, Y., et al., Force regulated conformational change of integrin αVβ3, Matrix Biology, 2016.
- Li, W., et al., Mild photothermal therapy/photodynamic therapy/chemotherapy of breast cancer by Lyp-1 modified Docetaxel/IR820 Co-loaded micelles, Biomaterials, 2016, 106, p. 119-133.
- Jiang, Y., et al., PEGylated Polyamidoamine dendrimer conjugated with tumor homing peptide as a potential targeted delivery system for glioma, Colloids and Surfaces B: Biointerfaces, 2016, 147, p. 242-249.
- Wu, L., et al., N-Terminal Modification with Pseudo-Bifunctional PEG-Hexadecane Markedly Improves the Pharmacological Profile of Human Growth Hormone, Molecular Pharmaceutics, 2015.
- Yao, H., et al., Enhanced blood–brain barrier penetration and glioma therapy mediated by a new peptide modified gene delivery system, Biomaterials, 2015, 37, P. 345-352.
- Jiao, Y., et al., A Functionalized Hollow Mesoporous Silica Nanoparticles‐Based Controlled Dual‐Drug Delivery System for Improved Tumor Cell Cytotoxicity, Particle & Particle Systems Characterization, 2015, V 32(2): 222-233.
- Morimatsu, M., et al., Visualizing the Interior Architecture of Focal Adhesions with High-Resolution Traction Maps, Nano Letters, 2015, 15(4): 2220-2228.
- Zheng, S., et al., Salvaging brain ischemia by increasing neuroprotectant uptake via nanoagonist mediated blood brain barrier permeability enhancement, Biomaterials, 2015, 66, P. 9-20.
- Zhou, Z., et al., Specific Conjugation of the Hinge Region for Homogeneous Preparation of Antibody Fragment-Drug Conjugate: A Case Study for Doxorubicin-PEG-anti-CD20 Fab′ Synthesis, Bioconjugate Chemistry, 2015.
- Lozeau, L. D., et al., Proposed Mechanisms of Tethered Antimicrobial Peptide Chrysophsin-1 as a Function of Tether Length Using QCM-D, The Journal of Physical Chemistry B, 2015, 119 (41), 13142-13151.
- Li, X., et al., D-SP5 Peptide-Modified Highly Branched Polyethylenimine for Gene Therapy of Gastric enocarcinoma, Bioconjugate Chemistry, 2015, 26 (8), 1494-1503.
- Choi, J.W., et al., Hepatoma targeting peptide conjugated bio-reducible polymer complexed with oncolytic adenovirus for cancer gene therapy, Journal of Controlled Release, 2015, 220B, P. 691-703.
- Ju, L., et al, Transport Regulation of Two-Dimensional Receptor-Ligand Association, Biophysical Journal, 2015, 108:7, P. 1773-1784.
- Wang, K., et al., Specific aptamer-conjugated mesoporous silica–carbon nanoparticles for HER2-targeted chemo-photothermal combined therapy, Acta Biomaterialia, 2015, 16, P. 196-205.
- Jiao, Y., et al., Tumor-Targeting Multifunctional Rattle-Type Theranostic Nanoparticles for MRI/NIRF Bimodal Imaging and Delivery of Hydrophobic Drugs, Small, 2015, 11: 1962–1974.
- Li, J., et al., Enhancement and wavelength-shifted emission of Cerenkov luminescence using multifunctional microspheres, Physics in Medicine and Biology, 2015, 60:2.
- Ren, H., et al., Peptide GE11–Polyethylene Glycol–Polyethylenimine for targeted gene delivery in laryngeal cancer, Medical Oncology, 2015, 32:185.
- Ma, P., et al., Targeted delivery of polyamidoamine-paclitaxel conjugate functionalized with anti-human epidermal growth factor receptor 2 trastuzumab. International Journal of Nanomedicine, 2015, 10:2173-2190.
- Chen, Y., et al., Highly effective antiangiogenesis via magnetic mesoporous silica-based siRNA vehicle targeting the VEGF gene for orthotopic ovarian cancer therapy. International Journal of Nanomedicine, 2015, 10:2579-2594.
- Liu, Y., et al., Gold nanorods/mesoporous silica-based nanocomposite as theranostic agents for targeting near-infrared imaging and photothermal therapy induced with laser. International Journal of Nanomedicine, 2015, 10:4747-4761.
- Look, J., et al., Ligand-Modified Human Serum Albumin Nanoparticles for Enhanced Gene Delivery, Molecular Pharmaceutics, 2015, 12 (9), 3202-3213.
- Yao, H., et al., Enhanced blood–brain barrier penetration and glioma therapy mediated by a new peptide modified gene delivery system, Biomaterials, 2015, 37, P. 345-352.
- Luo. B., et al., Novel lactoferrin-conjugated amphiphilic poly(aminoethyl ethylene phosphate)/poly(L-lactide) copolymer nanobubbles for tumor-targeting ultrasonic imaging. International Journal of Nanomedicine, 2015, 10:5805-5817.
- Sofla, F.J.I., et al., Specific gene delivery mediated by poly(ethylene glycol)-grafted polyamidoamine dendrimer modified with a novel HER2-targeting nanobody, Journal of Bioactive and Compatible Polymers, 2015, v. 30:2, 129-144.
- Raviv, L., et al., Mannosylated Polyion Complexes for In Vivo Gene Delivery into CD11c+ Dendritic Cells, Molecular Pharmaceutics, 2015, 12 (2), 453-462.
- Liu, Y., et al., Single peptide ligand-functionalized uniform hollow mesoporous silica nanoparticles achieving dual-targeting drug delivery to tumor cells and angiogenic blood vessel cells. International Journal of Nanomedicine, 2015, 10:1855-1867.
- Singh, A., et al., A hyaluronic acid-binding contact lens with enhanced water retention, Contact Lens and Anterior Eye, 2015, 38:2, P. 79-84.
- Gobaa, S., et al., Substrate elasticity modulates the responsiveness of mesenchymal stem cells to commitment cues, Integr. Biol., 2015, 7, 1135-1142.
- Yang, Z., et al., Dual-Ligand Modified Polymer-Lipid Hybrid Nanoparticles for Docetaxel Targeting Delivery to Her2/neu Overexpressed Human Breast Cancer Cells, Journal of Biomedical Nanotechnology, 2015, 11:8.
- Bhandari, H., et al., Evaluation of Bone Targeting Salmon Calcitonin Analogues in Rats Developing Osteoporosis and Adjuvant Arthritis, Current Drug Delivery, 2015, 12:1, pp. 98-107(10).
- Zeng, Y., et al., The conformational states of talin autoinhibition complex and its activation under forces, Science China Life Sciences, 2015, 58:7, pp 694-703.
- Fu, X., et al., Anchorage-dependent binding of integrin I-domain to adhesion ligands, J. Mol. Recognit., 2015, 28, 385–392.
- Li, M., et al., Investigating the Molecular Specific Interactions on Cell Surface Using Atomic Force Microscopy, Micro-and Nanomanipulation Tools, 2015.
- Chen, Y., et al., Fluorescence Biomembrane Force Probe: Concurrent Quantitation of Receptor-ligand Kinetics and Binding-induced Intracellular Signaling on a Single Cell, J. Vis. Exp., 2015, (102), e52975.
- Golani-Armon, A., et al., DC3-Decorated Polyplexes for Targeted Gene Delivery into Dendritic Cells, Bioconjugate Chemistry, 2015, 26 (2), 213-224.
- Wang, K., et al., Development of biodegradable polymeric implants of RGD-modified PEG-PAMAM-DOX conjugates for long-term intratumoral release, Drug Delivery, 2015, Vol. 22, Iss. 3.
- Braun, G.B., et al., Etchable plasmonic nanoparticle probes to image and quantify cellular internalization, Nature Materials, 2014, 13, p: 904–911
- Mei, L., et al., Increased tumor targeted delivery using a multistage liposome system functionalized with RGD, TAT and cleavable PEG. International Journal of Pharmaceutics, 2014, 468(1–2): p. 26-38.
- Pang, H.-B., et al., A free cysteine prolongs the half-life of a homing peptide and improves its tumor-penetrating activity. Journal of Controlled Release, 2014, 175: p. 48-53.
- Kong, X., et al., A novel multifunctional poly(amidoamine) dendrimeric delivery system with superior encapsulation capacity for targeted delivery of the chemotherapy drug 10-hydroxycamptothecin. International Journal of Pharmaceutics, 2014, 465(1–2): p. 378-387.
- Wang, F., et al., Efficient, dual-stimuli responsive cytosolic gene delivery using a RGD modified disulfide-linked polyethylenimine functionalized gold nanorod, Journal of Controlled Release, 2014, 196, p: 37-51.
- Fiore, V. F., et al., Dynamic catch of a Thy-1-alpha5beta1+syndecan-4 trimolecular complex, Nat Commun, 2014, 5: 4886.
- Zong, T., et al., Enhanced Glioma Targeting and Penetration by Dual-Targeting Liposome Co-modified with T7 and TAT, Journal of Pharmaceutical Sciences, 2014, 103(12): 3891-3901.
- Wang, K., et al., Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates, Biomaterials, 2014, 35:30, p. 8735-8747.
- Yang, Y., et al., Synthesis, Characterization and Biodistribution Studies of 125I-Radioiodinated di-PEGylated Bone Targeting Salmon Calcitonin Analogue in Healthy Rats, Pharmaceutical Research, 2014, 31:5, pp 1146-1157.
- Qin, L., et al., A dual‑targeting liposome conjugated with transferrin and arginine‑glycine‑aspartic acid peptide for glioma‑targeting therapy. Oncology Letters, 2014, 8, 2000-2006.
- Xiao, B., et al., Glycoprotein CD98 as a receptor for colitis-targeted delivery of nanoparticles, J. Mater. Chem. B, 2014, 2, 1499-1508.
- Wang, Y., et al., Synthesis of Core–Shell Graphitic Carbon@Silica Nanospheres with Dual-Ordered Mesopores for Cancer-Targeted Photothermochemotherapy, ACS Nano, 2014, 8 (8), 7870-7879.
- Wang, Y., et al., MRI-Visualized, Dual-Targeting, Combined Tumor Therapy Using Magnetic Graphene-Based Mesoporous Silica, Small, 2014, 10: 109–116.
- Li, M., et al., Nanoscale distribution of CD20 on B-cell lymphoma tumour cells and its potential role in the clinical efficacy of rituximab. Journal of Microscopy, 2014, 254: 19–30.
- Parreira, P., et al., Atomic force microscopy measurements reveal multiple bonds between Helicobacter pylori blood group antigen binding adhesin and Lewis b ligand, J. R. Soc. Interface, 2014, 11 20141040.
- Li, M., et al., AFM analysis of the multiple types of molecular interactions involved in rituximab lymphoma therapy on patient tumor cells and NK cells, Cellular Immunology, 2014, 290:2, P. 233-244.
- Kouchakzadeh, H., et al., Efficient loading and entrapment of tamoxifen in human serum albumin based nanoparticulate delivery system by a modified desolvation technique, Chemical Engineering Research and Design, 2014, 92:9, P. 1681-1692.
- Wang, J., et al., Retro-Inverso CendR Peptide-Mediated Polyethyleneimine for Intracranial Glioblastoma-Targeting Gene Therapy, Bioconjugate Chem., 2014, 25(2) p:414–423.
- Liu, Y., et al., A Bacteria Deriving Peptide Modified Dendrigraft Poly-l-lysines (DGL) Self-Assembling Nanoplatform for Targeted Gene Delivery, Molecular Pharmaceutics, 2014, 11 (10), 3330-3341.
- Tang, J., et al., Liposomes co-modified with cholesterol anchored cleavable PEG and octaarginines for tumor targeted drug delivery, Journal of Drug Targeting, 2014, 22:4.
- Zong, T., et al., Synergistic Dual-Ligand Doxorubicin Liposomes Improve Targeting and Therapeutic Efficacy of Brain Glioma in Animals, Molecular Pharmaceutics, 2014, 11 (7), 2346-2357.
- Gao, X., et al., Overcoming the blood–brain barrier for delivering drugs into the brain by using adenosine receptor nanoagonist. ACS nano, 2014, 8(4):3678-89.
- Xiao, B., et al., Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology, 2014, 146(5):1289-300.
- He, H. et al., The use of low molecular weight protamine chemical chimera to enhance monomeric insulin intestinal absorption, Biomaterials, 2013, 34(31), p: 7733-7743.
- Altintas, I., et al., Nanobody-albumin nanoparticles (NANAPs) for the delivery of a multikinase inhibitor 17864 to EGFR overexpressing tumor cells, Journal of Controlled Release, 2013, 165(2), p: 110-118.
- Loessner, D., et al., Hydrogel Microwell Arrays Allow the Assessment of Protease-Associated Enhancement of Cancer Cell Aggregation and Survival, Microarrays, 2013, 2, 208-227.
- Yan, F., et al., Transferrin-conjugated, fluorescein-loaded magnetic nanoparticles for targeted delivery across the blood–brain barrier, J Mater Sci: Mater Med, 2013, 24:2371–2379.
- Han, L., et al., Acid active receptor-specific peptide ligand for in vivo tumor-targeted delivery, Small, 2013, 9(21): 3647-3658.
- Allazetta, S., et al., Microfluidic Synthesis of Cell-Type-Specific Artificial Extracellular Matrix Hydrogels, Biomacromolecules, 2013, 14(4), pp 1122–1131.
- Cosson, S., et al., Patterning of cell-instructive hydrogels by hydrodynamic flow focusing, Lab on a Chip, 2013, 13(11):2099-105.
- Li, Mi, et al., Mapping CD20 molecules on the lymphoma cell surface using atomic force microscopy, Chinese Science Bulletin, 2013, 58.13 : 1516-1519.
- Li, Mi, et al. Imaging and measuring the biophysical properties of Fc gamma receptors on single macrophages using atomic force microscopy, Biochemical and biophysical research communications, 2013, 438.4 : 709-714.
- Xu, J., et al, Biodistribution and pharmacokinetics of EGFR-targeted thiolated gelatin nanoparticles following systemic administration in pancreatic tumor-bearing mice. Molecular pharmaceutics, 2013, 10(5):2031-44.
- Wang, J., et al., Targeted gene delivery to glioblastoma using a C-end rule RGERPPR peptide-functionalised polyethylenimine complex. International journal of pharmaceutics, 2013, 458(1):48-56.
- Li, Mi, et al., Nanoscale mapping and organization analysis of target proteins on cancer cells from B-cell lymphoma patients, Experimental cell research, 2013, 319.18 : 2812-2821.
- Ju L, et al., The N-terminal flanking region of the A1 domain regulates the force-dependent binding of von Willebrand factor to platelet glycoprotein Ibα, Journal of Biological Chemistry, 2013, 88(45):32289-301.
- Li, M., et al., Atomic force microscopy study of the antigen‐antibody binding force on patient cancer cells based on ROR1 fluorescence recognition. Journal of Molecular Recognition, 2013, 432-438.
- Wang, Y., et al., Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. Journal of the American Chemical Society, 2013, 135(12):4799-804.
- Li, J., et al., A choline derivate-modified nanoprobe for glioma diagnosis using MRI, Scientific reports, 2013, 3 .
- Agemy, L., et al., Proapoptotic peptide-mediated cancer therapy targeted to cell surface p32. Molecular Therapy, 2013, 21(12):2195.
- Ran, R., et al., Enhanced tumor accumulation and cellular uptake of liposomes modified with ether-bond linked cholesterol derivatives, Die Pharmazie-An International Journal of Pharmaceutical Sciences, 2013, 68.8 : 668-674.
- Sun, C., et al., Bifunctional PEGylated exenatide-amylinomimetic hybrids to treat metabolic disorders: an example of long-acting dual hormonal therapeutics, Journal of medicinal chemistry, 2013, 56.22 : 9328-9341.
- Huang, S., et al., Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis, ACS nano, 2013, 7.3, 2860-2871.
- Xiao, Y., Gold Nanorods Conjugated with Doxorubicin and cRGD for Combined Anticancer Drug Delivery and PET Imaging, Theranostics, 2012, 2(8):757-768.
- Blevins, K.S., et al., EphA2 targeting peptide tethered bioreducible poly(cystamine bisacrylamide–diamino hexane) for the delivery of therapeutic pCMV-RAE-1γ to pancreatic islets, Journal of Controlled Release, 2012, 158(1), p: 115-122.
- Zhou, J. et al., Octa-functional PLGA nanoparticles for targeted and efficient siRNA delivery to tumors, Biomaterials, 2012, 33(2) p: 583-591.
- Li, C., et al., Preparation, structural analysis and bioactivity of ribonuclease A-albumin conjugate: Tetra-conjugation or PEG as the linker. Journal of Biotechnology, 2012, 162(2–3): p. 283-288.
- Cai, M., et al., Direct evidence of lipid rafts by in situ atomic force microscopy, Small, 2012, 8(8): 1243-1250.
- Hakanson, M., et al., Controlled Breast Cancer Microarrays for the Deconvolution of Cellular Multilayering and Density Effects upon Drug Responses. PLoS ONE, 2012, 7(6): e40141.
- Zhan, C., et al., Co-delivery of TRAIL gene enhances the anti-glioblastoma effect of paclitaxel in vitro and in vivo, Journal of Controlled Release, 2012, 160:3, P. 630-636.
- Kouchakzadeh, H., et al., Attachment of an anti-MUC1 monoclonal antibody to 5-FU loaded BSA nanoparticles for active targeting of breast cancer cells, Hum Antibodies, 2012, 21(3-4):49-56.
- Vitaliano, G.D., et al., New clathrin-based nanoplatforms for magnetic resonance imaging. PloS one., 2012, 7(5):e35821.
- Yang, X., et al., cRGD-functionalized, DOX-conjugated, and 64Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials, 2011, 32(17): p. 4151-4160.
- Sadeqzadeh, E., et al., Combined MUC1-specific nanobody-tagged PEG-polyethylenimine polyplex targeting and transcriptional targeting of tBid transgene for directed killing of MUC1 over-expressing tumour cells, Journal of Controlled Release, 2011, 156:1, P. 85-91.
- Wang, Y.Y., et al., Introducing RGD Peptides on PHBV Films through PEG-Containing Cross-Linkers to Improve the Biocompatibility, Biomacromolecules, 2011, 12(3), p: 551–559.
- Han, L., et al., Plasmid pORF-hTRAIL and doxorubicin co-delivery targeting to tumor using peptide-conjugated polyamidoamine dendrimer, Biomaterials, 2011, 32:4, P. 1242-1252.
- Huang, S., et al., Dual targeting effect of Angiopep-2-modified, DNA-loaded nanoparticles for glioma, Biomaterials, 2011, 32:28, P. 6832-6838.
- Han, L., et al., Peptide-conjugated polyamidoamine dendrimer as a nanoscale tumor-targeted T1 magnetic resonance imaging contrast agent, Biomaterials, 2011, 32:11, P. 2989-2998.
- Li, M., et al., Detecting CD20-Rituximab interaction forces using AFM single-molecule force spectroscopy, Chinese Science Bulletin, 2011, 56:35, pp 3829-3835.
- Chandrawati, R., et al., Degradation of liposomal subcompartments in PEGylated capsosomes, Soft Matter, 2011, 7, 9638-9646.
- Zhang, L., et al., RGD-modified PEG–PAMAM–DOX conjugates: In vitro and in vivo studies for glioma, European Journal of Pharmaceutics and Biopharmaceutics, 2011, 79:2, P. 232-240.
- Zhu, S., et al., RGD-Modified PEG-PAMAM-DOX Conjugate: In Vitro and In Vivo Targeting to Both Tumor Neovascular Endothelial Cells and Tumor Cells, Adv. Mater., 2011, 23: H84–H89.
- Wang, G., et al., Bisphosphonate-coated BSA nanoparticles lack bone targeting after systemic administration. J Drug Target, 2010, 18(8): p. 611-26.
- Wei, C., et al., Forcing Switch from Short- to Intermediate- and Long-lived States of the αA Domain Generates LFA-1/ICAM-1 Catch Bonds, J Biol Chem., 2010, 285(46): 35967–35978.
- Gu, B., et al., Folate-PEG-CKK2-DTPA, A Potential Carrier for Lymph-Metastasized Tumor Targeting, Pharmaceutical Research, 2010, 27(5) p 933-942.
- Zhang, C., et al., Targeted minicircle DNA delivery using folate-poly(ethylene glycol)-polyethylenimine as non-viral carrier, Biomaterials, 2010, 31(23):6075-86.
- Kuai, R., et al., Efficient Delivery of Payload into Tumor Cells in a Controlled Manner by TAT and Thiolytic Cleavable PEG Co-Modified Liposomes, Molecular Pharmaceutics, 2010, 7 (5), 1816-1826.
- Zhang, H., et al., Multifunctional Peptide-PEG Intercalating Conjugates: Programmatic of Gene Delivery to the Blood-Brain Barrier, Pharmaceutical Research, 2010, 27:12, pp 2528-2543.
- Wang, Y.Y., et al., Cellular compatibility of RGD-modified chitosan nanofibers with aligned or random orientation, Biomedical Materials, 2010, 5:5.
- Agemy, L., et al., Nanoparticle-induced vascular blockade in human prostate cancer, Blood, 2010, 116 (15) 2847-2856.
- Shan, Y., et al., Locating the Band III protein in quasi-native cell membranes, Anal. Methods, 2010, 2, 805-808.
- Carroll, R.T., et al., Brain-targeted delivery of Tempol-loaded nanoparticles for neurological disorders, Journal of Drug Targeting, 2010, 18:9.
- Huang, R., et al., Targeted delivery of chlorotoxin-modified DNA-loaded nanoparticles to glioma via intravenous administration, Biomaterials, 2011, 32:9, P. 2399-2406.
- Zhang, H., et al., Efficient Transfection of Blood−Brain Barrier Endothelial Cells by Lipoplexes and Polyplexes in the Presence of Nuclear Targeting NLS-PEG-Acridine Conjugates, Bioconjugate Chemistry, 2009, 20 (1), 120-128.
- Schumacher, W.C., et al., Detection of Bacillus anthracis spores: comparison of quantum dot and organic dye labeling agents, Advanced Powder Technology, 2009, 20:5, P. 438-446.
- Zhang, H., et al., Efficient Nuclear Targeting by NLS-PEG-Acridine Conjugates in the Transfection of Blood-Brain Barrier Endothelial Cells with Lipoplexes and Polyplexes, Bioconjugate chemistry, 2009, 20(1):120-128.
- Jeong, K.J., et al., Interplay between Covalent and Physical Interactions within Environment Sensitive Hydrogels, Biomacromolecules, 2009, 10 (5), 1090-1099.
- Cosson, S., et al., Capturing Complex Protein Gradients on Biomimetic Hydrogels for Cell-Based Assays. Adv. Funct. Mater., 2009, 19: 3411–3419.
- Schumacher, W.C., et al., Development of Novel Fluorescence-based Methods for Detection of Bacillus Anthracis Spores, Ohio State University, 2008.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.