PEG products with additional MW may be made to order, please contact us for details
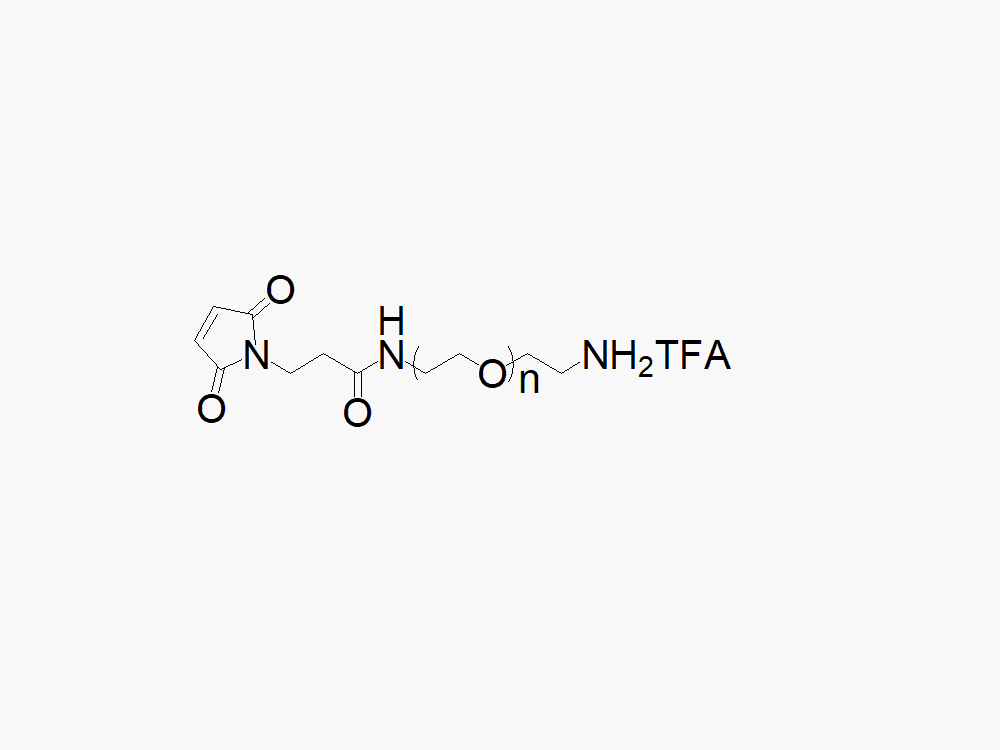
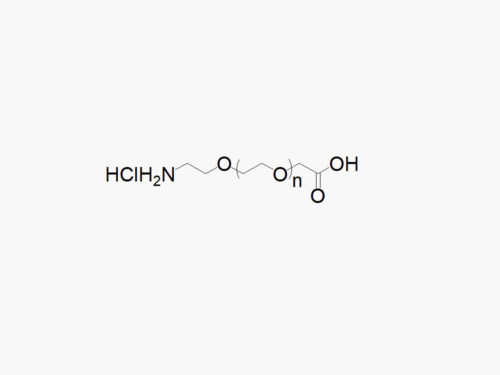
Maleimide PEG Amine, TFA Salt
$350.00 – $1,400.00
Description
High quality Maleimide PEG Amine, TFA salt (MAL-PEG-NH2TFA) with standard quality specification of ≥95% Substitution.
Heterobifunctional Maleimide PEG Amine products from JenKem Technology are generally employed as crosslinking agents or as spacers between two different chemical entities. The PEG moiety in the heterofunctional PEG derivatives provides water solubility, biocompatibility, and flexibility. Applications are especially geared towards the development of antibody drug conjugates (ADC’s).
Heterobifunctional PEGylation reagents with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability of custom PEGs.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Pham-Nguyen, O.V, et al., Complete breakdown of copper-free clickable doxorubicin nanoclusters for real-time tumor proliferation tracking, Chemical Engineering Journal, 468, 2023.
- Xi, X., Ligand-installed polymeric nanocarriers for combination chemotherapy of EGFR-positive ovarian cancer,Journal of Controlled Release, 360, 2023.
- Galindo-Camacho, R. M., et al., Cell penetrating peptides-functionalized Licochalcone-A-loaded PLGA nanoparticles for ocular inflammatory diseases: Evaluation of in vitro anti-proliferative effects, stabilization by freeze-drying and characterization of an in-situ forming gel, International Journal of Pharmaceutics, 2023, V. 639.
- Sanna, V., et al., Development of targeted nanoparticles loaded with antiviral drugs for SARS-CoV-2 inhibition, European Journal of Medicinal Chemistry, 2022,114121.
- Distasio, N, et al., VCAM‐1‐Targeted Gene Delivery Nanoparticles Localize to Inflamed Endothelial Cells and Atherosclerotic Plaques. Advanced Therapeutics. 2021, 4(2):2000196.
- Zhang, H., et al., Development of curcumin-loaded zein nanoparticles for transport across the blood–brain barrier and inhibition of glioblastoma cell growth. Biomaterials Science. 2021.
- Co, CM, et al., Click chemistry-based pre-targeting cell delivery for cartilage regeneration. Regenerative Biomaterials. 2021, 8(3).
- Yang, J, et al., Hierarchically Responsive Tumor‐Microenvironment‐Activated Nano‐Artificial Virus (TMAN) for Precise Exogenous and Endogenous Apoptosis Coactivation. Advanced Functional Materials. 2021, 2104423.
- Lv, F., et al., Enhanced mucosal penetration and efficient inhibition efficacy against cervical cancer of PEGylated docetaxel nanocrystals by TAT modification, Journal of Controlled Release, 2021, V. 336, P. 572-582.
- Chen, Q., et al., Deep tumor‐penetrated nanosystem eliminates cancer stem cell for highly efficient liver cancer therapy, Chemical Engineering Journal, 2021, V. 421 (2).
- Xia, C., et al., Redox-responsive nanoassembly restrained myeloid-derived suppressor cells recruitment through autophagy-involved lactate dehydrogenase A silencing for enhanced cancer immunochemotherapy, Journal of Controlled Release, 2021, V. 335, P. 557-574.
- Poli, H., et al., Optimisation of alendronate conjugation to polyethylene glycol for functionalisation of biopolymers and nanoparticles, European Polymer Journal, 2021, V. 155.
- Huo, T., et al., Versatile hollow COF nanospheres via manipulating transferrin corona for precise glioma-targeted drug delivery, Biomaterials, 2020, 260, 120305.
- Yang, S., et al., Virus-esque nucleus-targeting nanoparticles deliver trojan plasmid for release of anti-tumor shuttle protein, Journal of Controlled Release, 2020, V. 320, P. 253-264.
- Huang, Z.G., et al., RGD-modified PEGylated paclitaxel nanocrystals with enhanced stability and tumor-targeting capability, International journal of pharmaceutics, 2019, 556:217-25.
- Khondee, S., et al., Doxorubicin-Loaded Micelle Targeting MUC1: A Potential Therapeutic for MUC1 Triple Negative Breast Cancer Treatment, Current drug delivery, 2018, 15(3), pp.406-416.
- Yu, X., et al., Activatable Protein Nanoparticles for Targeted Delivery of Therapeutic Peptides, Advanced Materials, 2018.
- Wu, X., et al., A Cascade‐Targeting Nanocapsule for Enhanced Photothermal Tumor Therapy with Aid of Autophagy Inhibition, Advanced healthcare materials, 2018, p.1800121.
- Li, H., et al., Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma, International Journal of Biological Macromolecules, 2018, V. 107A, P. 204-211.
- Jiang, Y., et al., TEMPO-oxidized starch nanoassemblies of negligible toxicity compared with polyacrylic acids for high performance anti-cancer therapy, International Journal of Pharmaceutics, 2018, V. 547 (1–2), p. 520-529.
- Chen, G., et al., NIR-induced spatiotemporally controlled gene silencing by upconversion nanoparticle-based siRNA nanocarrier, Journal of Controlled Release, 2018, V. 282, P. 148-155.
- Yang, J., et al., A positron emission tomography image-guidable unimolecular micelle nanoplatform for cancer theranostic applications, Acta Biomaterialia, 2018, V. 79, P. 306-316.
- Verbeke, C. S., et al., Multicomponent Injectable Hydrogels for Antigen-Specific Tolerogenic Immune Modulation, Adv. Healthcare Mater., 2017, 6.
- Peng, H.B., et al., Nuclear-Targeted Multifunctional Magnetic Nanoparticles for Photothermal Therapy, Adv. Healthcare Mater., 2017, 6 (7).
- Clawson, G.A., et al., A Cholecystokinin B Receptor-Specific DNA Aptamer for Targeting Pancreatic Ductal Adenocarcinoma, Nucleic acid therapeutics, 2017, 27(1):23-35.
- Chen, G., et al., Tumor-targeted pH/redox dual-sensitive unimolecular nanoparticles for efficient siRNA delivery. Journal of Controlled Release, 2017.
- Wang, Y., et al., Carboplatin‐Complexed and cRGD‐Conjugated Unimolecular Nanoparticles for Targeted Ovarian Cancer Therapy, Macromolecular bioscience, 2017, 17(5).
- Chai, Z., et al., A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery, Journal of Controlled Release, 2017, V. 264, P. 102-111.
- Zhao, L., et al., Computational design of peptide-Au cluster probe for sensitive detection of α IIb β 3 integrin, Nanoscale, 2016, 8(7):4203-8.
- Xue, Y., et al., Preventing diet-induced obesity in mice by adipose tissue transformation and angiogenesis using targeted nanoparticles. Proceedings of the National Academy of Sciences, 2016.
- Ding, H., et al, HER2-positive breast cancer targeting and treatment by a peptide-conjugated mini nanodrug, Nanomedicine: Nanotechnology, Biology and Medicine, 2016.
- Li, L., et al., Multifunctional “core-shell” nanoparticles-based gene delivery for treatment of aggressive melanoma, Biomaterials, 2016, V. 111, p. 124-137
- Song, H., et al., Acid-responsive PEGylated doxorubicin prodrug nanoparticles for neuropilin-1 receptor-mediated targeted drug delivery, Colloids and Surfaces B: Biointerfaces, 2015, V. 136, P. 365-374.
- Shirbin, S,J., et al., Cisplatin-Induced Formation of Biocompatible and Biodegradable Polypeptide-Based Vesicles for Targeted Anticancer Drug Delivery, Biomacromolecules, 2015, 16 (8), 2463-2474.
- Liang, D.S., et al., Tumor-specific penetrating peptides-functionalized hyaluronic acid-d-α-tocopheryl succinate based nanoparticles for multi-task delivery to invasive cancers, Biomaterials, 2015, V. 71, P. 11-23.
- Vasconcelos, A., et al., Conjugation of cell-penetrating peptides with poly(lactic-co-glycolic acid)-polyethylene glycol nanoparticles improves ocular drug delivery, Int J Nanomedicine, 2015, 10: 609–631.
- Jian, W.-H., et al., Indocyanine Green-Encapsulated Hybrid Polymeric Nanomicelles for Photothermal Cancer Therapy, Langmuir, 2015, 31 (22), 6202-6210.
- Temme, S., et al., Noninvasive Imaging of Early Venous Thrombosis by 19F Magnetic Resonance Imaging With Targeted Perfluorocarbon Nanoemulsions, 2015, 131: 1405-1414.
- Na, Y., et al., Potent antitumor effect of neurotensin receptor-targeted oncolytic adenovirus co-expressing decorin and Wnt antagonist in an orthotopic pancreatic tumor model, Journal of Controlled Release, 2015, V. 220 B, P. 766-782.
- Bai, M.-Y. and S.-Z. Liu, A simple and general method for preparing antibody-PEG-PLGA sub-micron particles using electrospray technique: An in vitro study of targeted delivery of cisplatin to ovarian cancer cells. Colloids and Surfaces B: Biointerfaces, 2014, p: 346-353.
- Shin, M.C., et al., Combination of antibody targeting and PTD-mediated intracellular toxin delivery for colorectal cancer therapy, Journal of Controlled Release, 194, 2014, p: 197-210.
- Joachimiak, L.A., et al., The Structural Basis of Substrate Recognition by the Eukaryotic Chaperonin TRiC/CCT, Cell, 2014, 159(5), p: 1042-1055
- Huang, Y.-F., et al., pH-Responsive Hierarchical Transformation of Charged Lipid Assemblies within Polyelectrolyte Gel Layers with Applications for Controlled Drug Release and MR Imaging Contrast, J. Mater. Chem. B, 2014, 2, 4988-4992.
- Liu, Y., et al., A Bacteria Deriving Peptide Modified Dendrigraft Poly-l-lysines (DGL) Self-Assembling Nanoplatform for Targeted Gene Delivery, Molecular Pharmaceutics, 2014, 11 (10), 3330-3341.
- Yang, Z., et al., Multifunctional non-viral gene vectors with enhanced stability, improved cellular and nuclear uptake capability, and increased transfection efficiency, Nanoscale, 2014, 6, 10193-10206.
- Tao, C., et al., Development and characterization of GRGDSPC-modified poly(lactide-co-glycolide acid) porous microspheres incorporated with protein-loaded chitosan microspheres for bone tissue engineering, Colloids and Surfaces B: Biointerfaces, 2014, V. 122, P. 439-446.
- Qin, Y., et al., Liposomes formulated with fMLP-modified cholesterol for enhancing drug concentration at inflammatory sites, Journal of Drug Targeting, 2014, V. 22:2.
- Chiang W.H., et al., Functionalized polymersomes with outlayered polyelectrolyte gels for potential tumor-targeted delivery of multimodal therapies and MR imaging, J Control Release, 2013, 168(3):280-8.
- Huang, Q., et al., PEG as a spacer arm markedly increases the immunogenicity of meningococcal group Y polysaccharide conjugate vaccine, Journal of Controlled Release, 2013, 172(1):382-9.
- Gao, X., et al., Up-regulating blood brain barrier permeability of nanoparticles via multivalent effect, Pharmaceutical research, 2013, 30.10 : 2538-2548.
- Lei, Y., et al. Glutathione-sensitive RGD-poly(ethylene glycol)-SS-polyethylenimine for intracranial glioblastoma targeted gene delivery. J. Gene Med., 2013, 15: 291–305.
- Wagh, A., et al., Polymeric nanoparticles with sequential and multiple FRET cascade mechanisms for multicolor and multiplexed imaging, Small, 2013, 9.12 : 2129-2139.
- Wagh, A., Development of Biocompatible Polymeric Nanoparticles for in Vivo NIR and FRET Imaging, Bioconjugate Chem., 2012, 23(5), pp 981–992.
- Xiao, Y., et al., Multifunctional unimolecular micelles for cancer-targeted drug delivery and positron emission tomography imaging, Biomaterials, 2012, 33(11), p: 3071-3082.
- Amphiphilic block copolymer; Positron emission tomography (PET); Cclic arginine-glycine-aspartic acid (cRGD) peptide. Pi-Ping Lv, et al., Targeted Delivery of Insoluble Cargo (Paclitaxel) by PEGylated Chitosan Nanoparticles Grafted with Arg-Gly-Asp (RGD), Mol. Pharmaceutics, 2012, 9(6) p: 1736–1747.
- Alibeik, S., et al., Modification of Polyurethane with Polyethylene Glycol–Corn Trypsin Inhibitor for Inhibition of Factor Xlla in Blood Contact, Journal of Biomaterials Science, Polymer Edition, 2012, 23:15, 1981-1993.
- Milane, L., et al., Development of EGFR-Targeted Polymer Blend Nanocarriers for Combination Paclitaxel/Lonidamine Delivery To Treat Multi-Drug Resistance in Human Breast and Ovarian Tumor Cells, Molecular Pharmaceutics, 2011, 8 (1), 185-203.
- Qin, Y., et al., Liposome formulated with TAT-modified cholesterol for enhancing the brain delivery, International Journal of Pharmaceutics, 2011, 419(1–2), p:85-95.
- Milane, L., et al., Therapeutic Efficacy and Safety of Paclitaxel/Lonidamine Loaded EGFR-Targeted Nanoparticles for the Treatment of Multi-Drug Resistant Cancer, PLoS ONE, 2011, 6(9), p. e24075.
- Alibeik, S., et al., Surface modification with polyethylene glycol–corn trypsin inhibitor conjugate to inhibit the contact factor pathway on blood-contacting surfaces, Acta Biomaterialia, 2011, V. 7, Iss. 12, P. 4177-4186.
- Milane, L., et al., Pharmacokinetics and biodistribution of lonidamine/paclitaxel loaded, EGFR-targeted nanoparticles in an orthotopic animal model of multi-drug resistant breast cancer, Nanomedicine: Nanotechnology, Biology and Medicine, 2011, V. 7:4, P. 435-444.
- Milane, L., et al., Biodistribution and Pharmacokinetic Analysis of Combination Lonidamine and Paclitaxel Delivery in an Orthotopic Animal Model of Multi-drug Resistant Breast Cancer Using EGFR-Targeted Polymeric Nanoparticles, Nanomedicine : nanotechnology, biology, and medicine, 2011, 7(4):435-444.
- Guopei Luo, G., et al., LyP-1-conjugated nanoparticles for targeting drug delivery to lymphatic metastatic tumors, International Journal of Pharmaceutics, 2010, 385(1–2), p: 150-156.
- Yang, X., et al., Multifunctional stable and pH-responsive polymer vesicles formed by heterofunctional triblock copolymer for targeted anticancer drug delivery and ultrasensitive MR imaging, ACS Nano, 2010, 4(11):6805-17.
- Yang, X., et al., Multifunctional SPIO/DOX-loaded wormlike polymer vesicles for cancer therapy and MR imaging, Biomaterials, 2010, V. 31:34, P. 9065-9073.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.