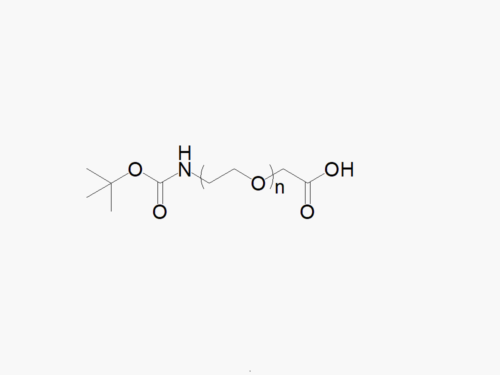
PEG products with additional MW may be made to order, please contact us for details
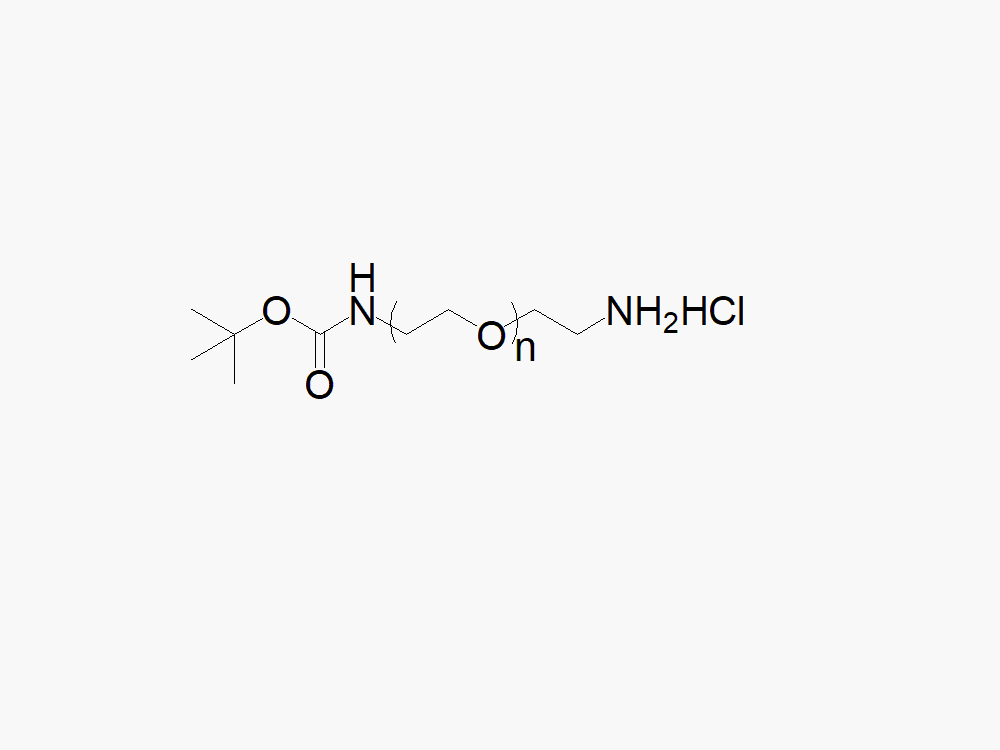
t-Boc Amine PEG Amine, HCl Salt
$280.00 – $1,120.00
Description
High quality t-Boc Amine PEG Amine, HCl Salt with standard quality specification of ≥95% Substitution.
Heterobifunctional t-Boc Amine PEG Amine products with TBOC protected amine, and amine HCl salt, from JenKem Technology, are generally employed as crosslinking agents or as spacers between two different chemical entities. The PEG moiety in the heterofunctional PEG derivatives provides water solubility, biocompatibility, and flexibility. Applications are especially geared towards the development of antibody drug conjugates (ADC’s).
Heterobifunctional PEGylation reagents with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability of custom PEGs.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
Note: Starting September 2017, the item code for these products has been changed to TBOCNH-PEG-NH2HCl. This is only a name change implemented to better reflect the chemical structure of TBOC-PEG-NH2HCl.
References:
- Han, Y., et al., Effective oral delivery of Exenatide-Zn2+ complex through distal ileum-targeted double layers nanocarriers modified with deoxycholic acid and glycocholic acid in diabetes therapy, Biomaterials, 2021, V. 275.
- Zhang, X., et al., Inhibiting PI3 kinase-γ in both myeloid and plasma cells remodels the suppressive tumor microenvironment in desmoplastic tumors, Journal of Controlled Release, 2019, 309, p. 173-180.
- Ai, F., et al., An upconversion nanoplatform with extracellular pH-driven tumor-targeting ability for improved photodynamic therapy, Nanoscale, 2018, 10(9), pp.4432-4441.
- Huo, M., et al., Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment, Journal of Controlled Release, 2017, V. 245, P. 81-94.
- Gajbhiye, K.R., et al., Ascorbic acid tethered polymeric nanoparticles enable efficient brain delivery of galantamine: An in vitro-in vivo study, Scientific Reports, 2017, 7: 11086.
- Li, Y., et al., A graphene quantum dot (GQD) nanosystem with redox-triggered cleavable PEG shell facilitating selective activation of the photosensitiser for photodynamic therapy, RSC Adv., 2016, 6, 6516-6522.
- Zhang, X., et al., Multimodal Upconversion Nanoplatform with a Mitochondria-Targeted Property for Improved Photodynamic Therapy of Cancer Cells. Inorganic chemistry, 2016, 55(8):3872-80.
- Zhao, Y., et al., Nanoparticle delivery of CDDO-Me remodels the tumor microenvironment and enhances vaccine therapy for melanoma, Biomaterials, 2015, V. 68, P. 54-66.
- Li, H., et al., Dual MMP7-Proximity-Activated and Folate Receptor-Targeted Nanoparticles for siRNA Delivery, Biomacromolecules, 2015, 16 (1), p: 192–201.
- Liu, J., et al., Integrin-targeted pH-responsive micelles for enhanced efficiency of anticancer treatment in vitro and in vivo, Nanoscale, 2015, 7, 4451-4460.
- Baker, D.W., et al., Development of optical probes for in vivo imaging of polarized macrophages during foreign body reactions. Acta Biomaterialia, 2014, 10(7): p. 2945-2955.
- Hsu, H.-J., et al., Poly(ethylene glycol) Corona Chain Length Controls End-Group-Dependent Cell Interactions of Dendron Micelles, Macromolecules, 2014, 47 (19), pp 6911–6918.
- Miao, L., et al., Nanoparticles with Precise Ratiometric Co-Loading and Co-Delivery of Gemcitabine Monophosphate and Cisplatin for Treatment of Bladder Cancer, Adv. Funct. Mater., 2014, 24: 6601–6611.
- Guo, S., et al., Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS nano, 2014, 8(5):4996-5009.
- Zhou, J., et al., In vivo evaluation of medical device-associated inflammation using a macrophage-specific positron emission tomography (PET) imaging probe, Bioorganic & Medicinal Chemistry Letters, 2013, 23(7), p: 2044-2047.
- Baker, D.W., The Pivotal Role Of Fibrocytes On Foreign Body Reactions, UTA, 2013.
- Li, D., et al., A novel chlorin–PEG–folate conjugate with higher water solubility, lower cytotoxicity, better tumor targeting and photodynamic activity, Journal of Photochemistry and Photobiology B: Biology, 2013, 127, 5, p. 28-37.
- Cao, P., et al., Improving Lanthanide Nanocrystal Colloidal Stability in Competitive Aqueous Buffer Solutions using Multivalent PEG-Phosphonate Ligands, Langmuir, 2012, 28(35), pp 12861–12870
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.