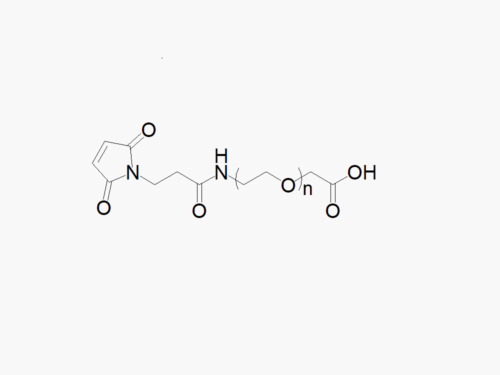
PEG products with additional MW may be made to order, please contact us for details
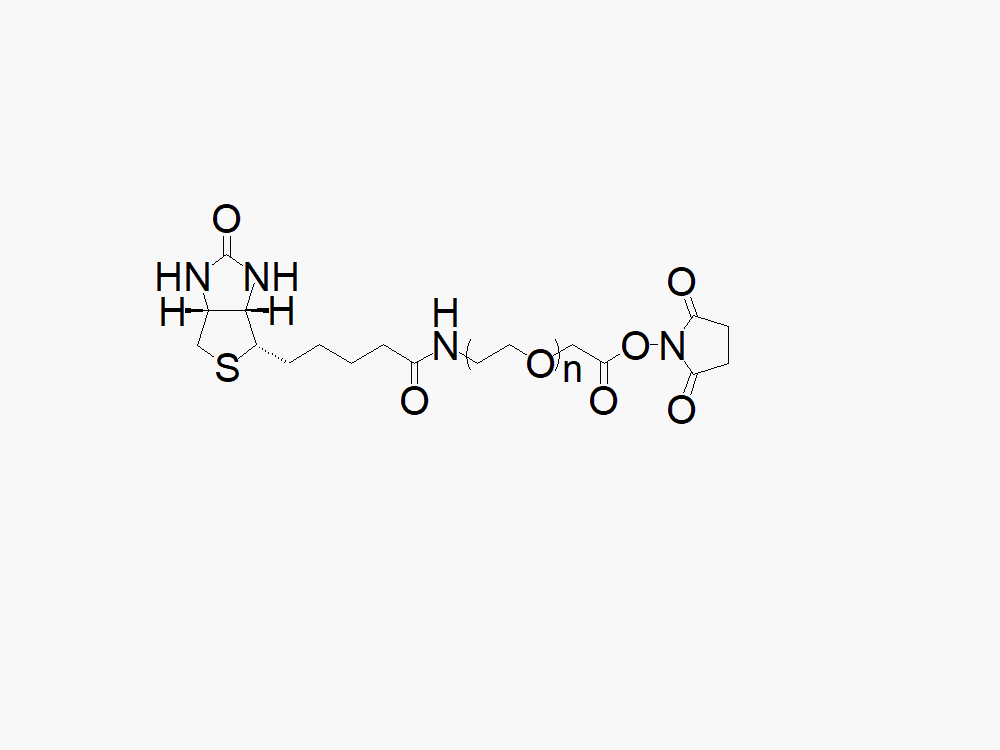
Biotin PEG Succinimidyl Carboxymethyl Ester
$500.00 – $2,000.00
Description
High quality Biotin PEG Succinimidyl Carboxymethyl Ester with a standard quality specification of >90% Substitution.
Heterobifunctional Biotin PEG Succinimidyl Carboxymethyl Ester (SCM) products from JenKem Technology are generally employed as crosslinking agents or as spacers between two different chemical entities. The PEG moiety in the heterofunctional biotinylated PEG derivatives provides water solubility, biocompatibility, and flexibility. Applications are especially geared towards antibody drug conjugation (ADC development).
Heterobifunctional PEGylation reagents with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability of custom PEGs.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Rosado, AM, et al., Memory in Repetitive Protein–Protein Interaction Series—in Memory of the Late Professor Robert M. Nerem. bioRxiv. 2022.
- Narvaez-Ortiz, H. Y., et al., Unconcerted conformational changes in Arp2/3 complex integrate multiple activating signals to assemble functional actin networks, Current Biology, 2022, 32(5).
- Balzer, C. J., et al., Single-Turnover Activation of Arp2/3 Complex by Dip1 May Balance Nucleation of Linear versus Branched Actin Filaments, Current Biology, 2019.
- Balzer, C.J., et al., Dip1 Co-opts Features of Branching Nucleation to Create Linear Actin Filaments that Activate WASP-Bound Arp2/3 Complex, Current Biology, 2018, 28(23), P. 3886-3891.
- Li, M., Mapping Membrane Proteins on Cell Surface by AFM, Investigations of Cellular and Molecular Biophysical Properties by Atomic Force Microscopy, Nanorobotics, 2018, pp. 65-77.
- Yu, Y., et al., A new NIR-triggered DOX and ICG co-delivery system for enhanced multidrug resistant cancer treatment through simultaneous chemo/photothermal/photodynamic therapy, Acta Biomaterialia, 2017.
- Son, Y.J., et al., Electrospun Nanofibrous Sheets for Selective Cell Capturing in Continuous Flow in Microchannels, Biomacromolecules, 2016.
- Zhang, W., et al., pH and near-infrared light dual-stimuli responsive drug delivery using DNA-conjugated gold nanorods for effective treatment of multidrug resistant cancer cells. Journal of Controlled Release, 2016, 232:9-19.
- Li, M., et al., Rapid recognition and functional analysis of membrane proteins on human cancer cells using atomic force microscopy, Journal of Immunological Methods, 2016.
- Chen, Y., et al., Force regulated conformational change of integrin αVβ3, Matrix Biology, 2016.
- Oztug Durer, Z.A., et al., Metavinculin Tunes the Flexibility and the Architecture of Vinculin-Induced Bundles of Actin Filaments, Journal of Molecular Biology, 2015, 427:17, P. 2782-2798.
- Rasson, A.S., et al., Filament Assembly by Spire: Key Residues and Concerted Actin Binding, Journal of Molecular Biology, 2015, 427 (4), p: 824-839.
- Chen, Y., et al., Fluorescence Biomembrane Force Probe: Concurrent Quantitation of Receptor-ligand Kinetics and Binding-induced Intracellular Signaling on a Single Cell, J. Vis. Exp., 2015, 102, e52975.
- Leiske, D.L., et al., Single-molecule enzymology based on the principle of the Millikan oil drop experiment, Analytical Biochemistry, 2014, 448, p: 30-37.
- Petek, N.A. and R.D. Mullins, Chapter Two – Bacterial Actin-Like Proteins: Purification and Characterization of Self-Assembly Properties, in Methods in Enzymology, 2014, p. 19-34.
- Zhao, G., et al., Functional PEG–PAMAM-Tetraphosphonate Capped NaLnF4 Nanoparticles and their Colloidal Stability in Phosphate Buffer. Langmuir, 2014, 30(23): p. 6980-6989.
- Kisley, L., et al., Extending single molecule fluorescence observation time by amplitude-modulated excitation, Methods Appl. Fluoresc., 2013, 1 037001, 7pp.
- Hansen, S.D, et al., Cytoplasmic actin: purification and single molecule assembly assays. InAdhesion Protein Protocols, 2013, pp. 145-170.
- Scott, M.A., Ultra-rapid 2-D and 3-D laser microprinting of proteins. Diss. Massachusetts Institute of Technology, 2013.
- Bor, B., et al., Autoinhibition of the formin Cappuccino in the absence of canonical autoinhibitory domains, Mol. Biol. Cell, 2012, 23(19) 3801-3813.
- Scott, M.A., Ultra-rapid laser protein micropatterning: screening for directed polarization of single neurons, Lab Chip, 2011.
- Hansen, S.D., et al., VASP is a processive actin polymerase that requires monomeric actin for barbed end association, J. Cell Biol., 2010, 191(3) p: 571–584.
- Andoy, N.M., et al., Single-Molecule Study of Metalloregulator CueR-DNA Interactions Using Engineered Holliday Junctions, Biophysical Journal, 97(3), 2009, p: 844-852.
- Chen, X., et al., Using Aptamer-Conjugated Fluorescence Resonance Energy Transfer Nanoparticles for Multiplexed Cancer Cell Monitoring, Analytical Chemistry, 2009, 81(16) p: 7009–7014.
- Ensign, A.E., Studies of Horse Heart Cytochrome c Folding, University of Rochester, 2009.
- Ochs, C.J., et al., Low-Fouling, Biofunctionalized, and Biodegradable Click Capsules, Biomacromolecules, 2008, 9(12) p: 3389–3396.
Note: Starting July 2016, Biotin PEG Succinimidyl Carboxymethyl Ester is the new name of the product Biotin PEG NHS Ester (MW 2000 (BIOTIN-PEG2000-NHS), MW 3500 (BIOTIN-PEG3500-NHS), MW 5000 (BIOTIN-PEG5000-NHS) and MW 7500 (BIOTIN-PEG7500-NHS)).
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.