“Novel Cell-Penetrating Drug Delivery System for siRNA” poster was presented by JenKem Technology and Tianjin Medical University at the 2018 TIDES meeting.

Experience has shown that siRNA drugs, while potentially very useful, are easily degraded by nucleases in vivo, and their relatively high molecular weight, negative charge and hydrophilicity inhibit their ability to permeate through cell membranes
To circumvent these deficiencies, a novel drug delivery system consisting of a cell penetrating peptide linked to activated PEGs (polyethylene glycol polymers) has been developed for siRNA delivery.
The addition of the PEG improves the drug’s bioavailability by preventing the self-assembly and formation of intra-molecular hairpin structures between the cell penetrating peptide and siRNA .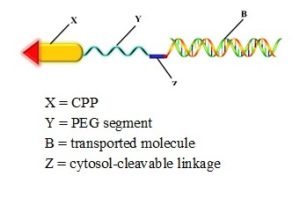
In vitro findings confirmed that our novel LMWP-PEG drug delivery system yields successful cellular uptake of siRNA and high gene-silencing efficacy.
LMWP-PEGs can be employed to deliver oligonucleotides for gene therapy, and other drug molecules that cannot penetrate the cell membrane on their own. For information please contact us.
References:
1. Zhao, X., et al., Novel Cell-Penetrating Drug Delivery System for siRNA, Poster presentation at 2018 TIDES.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.
