PEG products with additional MW may be made to order, please contact us for details
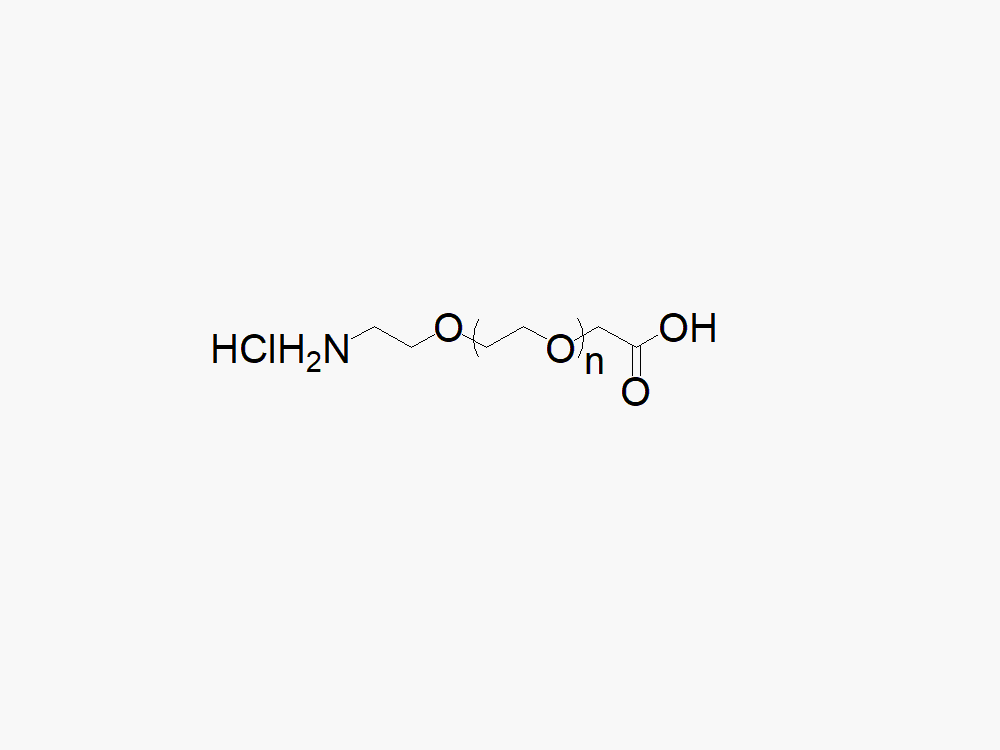
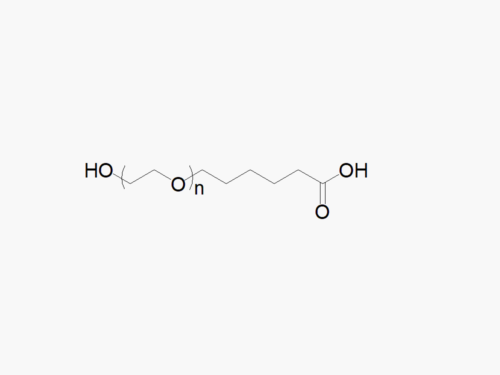
Amine PEG Acetic Acid, HCl Salt
$280.00 – $1,400.00
Description
High quality Amine PEG Acetic Acid, HCl Salt with standard quality specification of ≥95% Substitution.
Heterobifunctional Amine PEG Acetic Acid products from JenKem Technology are generally employed as crosslinking agents or as spacers between two different chemical entities. The PEG moiety in the heterobifunctional PEG derivatives provides water solubility, biocompatibility, and flexibility. Applications are especially geared towards the development of antibody drug conjugates (ADC’s).
Heterobifunctional PEGylation products with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability of custom PEGs.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Saeinasab, M., et al., Tumor-targeted delivery of SNHG15 siRNA using a ZIF-8 nanoplatform: Towards a more effective prostate cancer therapy, International Journal of Biological Macromolecules, 2024.
- Shirasu, T., et al., Neointima abating and endothelium preserving — An adventitia-localized nanoformulation to inhibit the epigenetic writer DOT1L, Biomaterials, 301, 2023.
- Poellmann, M. J., et al., Circulating tumor cell abundance in head and neck squamous cell carcinoma decreases with successful chemoradiation and cetuximab treatment, Cancer Letters, 2023, V. 562.
- Poellmann, MJ., et al., Nanotechnology and machine learning enable circulating tumor cells as a reliable biomarker for radiotherapy responses of gastrointestinal cancer patients, Biosensors and Bioelectronics, 2023: 115117.
- Guo, L.Y., et al., Skin-safe nanophotosensitizers with highly-controlled synthesized polydopamine shell for synergetic chemo-photodynamic therapy, Journal of Colloid and Interface Science, 2022, 616, pp.81-92.
- Damrongrak, K., et al., Delivery of acetogenin-enriched Annona muricata Linn leaf extract by folic acid-conjugated and triphenylphosphonium-conjugated poly (glycerol adipate) nanoparticles to enhance toxicity against ovarian cancer cells, International Journal of Pharmaceutics, 2022, 121636.
- Sanna, V., et al., Development of targeted nanoparticles loaded with antiviral drugs for SARS-CoV-2 inhibition, European Journal of Medicinal Chemistry, 2022,114121.
- Sheng, Q, et al., Comprehensively enhanced delivery cascade by transformable beaded nanofibrils for pancreatic cancer therapy. Nanoscale. 2021.
- Deng, M, et al., pH-Triggered Copper-Free Click Reaction-Mediated Micelle Aggregation for Enhanced Tumor Retention and Elevated Immuno–Chemotherapy against Melanoma. ACS Applied Materials & Interfaces. 2021, 13(15):18033-46.
- Lumen, D., et al., Investigation of silicon nanoparticles produced by centrifuge chemical vapor deposition for applications in therapy and diagnostics, European Journal of Pharmaceutics and Biopharmaceutics, 2021, V. 158, P. 254-265.
- Poojari, R. et al., Distinct stratification of normal liver, hepatocellular carcinoma (HCC), and anticancer nanomedicine-treated- tumor tissues by Raman fingerprinting for HCC therapeutic monitoring, Nanomedicine: Nanotechnology, Biology and Medicine, 2021, V. 33.
- Li, Z., et al., Nanoparticle depots for controlled and sustained gene delivery, Journal of Controlled Release, 2020.
- Wang, Z.-L., et al., Capture of Circulating Tumor Cells by Hydrogel-Nanofiber Substrate, Chinese Journal of Analytical Chemistry, 2019, 47 (8), p. 1162-1169.
- Terracciano, M., et al., In Vivo Toxicity Assessment of Hybrid Diatomite Nanovectors Using Hydra vulgaris as a Model System, Advanced Biosystems, 2019.
- Lu, J., et al., Fabrication of thermo-and pH-sensitive cellulose nanofibrils-reinforced hydrogel with biomass nanoparticles, Carbohydrate Polymers, 2019.
- Terracciano, M., et al., Gold decorated porous biosilica nanodevices for advanced medicine, Nanotechnology, 2018, 29(23), p.235601.
- Kushwah, V., et al., Implication of linker length on cell cytotoxicity, pharmacokinetic and toxicity profile of gemcitabine-docetaxel combinatorial dual drug conjugate, International Journal of Pharmaceutics, 2018, V. 548 (1), P. 357-374.
- Alibolandi, M., et al., Tetrac-conjugated polymersomes for integrin-targeted delivery of camptothecin to colon adenocarcinoma in vitro and in vivo, International Journal of Pharmaceutics, 2017, V. 532 (1), p. 581-594.
- Xu, L., et al., Synthesis and Application of Injectable Bioorthogonal Dendrimer Hydrogels for Local Drug Delivery, ACS Biomaterials Science & Engineering, 2017.
- Zhao, L., et al., An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. Journal of Controlled Release, 2017.
- Sanna, V., et al., Targeted nanoparticles encapsulating (−)-epigallocatechin-3-gallate for prostate cancer prevention and therapy, Scientific Reports, 2017, 7:41573.
- Alpsoy, L., et al., Synthesis and Characterization of Carboxylated Luteolin (CL)-Functionalized SPION, Journal of Superconductivity and Novel Magnetism, 2017.
- Clawson, G.A., et al., A Cholecystokinin B Receptor-Specific DNA Aptamer for Targeting Pancreatic Ductal Adenocarcinoma, Nucleic acid therapeutics, 2017, 27(1):23-35.
- Ai, P., et al., The relative length of dual-target conjugated on iron oxide nanoparticles plays a role in brain glioma targeting, RSC Advances, 2017, 7(32):19954-9.
- Gao, D., et al., Targeted Ultrasound-Triggered Phase Transition Nanodroplets for Her2-Overexpressing Breast Cancer Diagnosis and Gene Transfection. Molecular Pharmaceutics, 2017, 14(4):984-98.
- Qi, Q., et al., Spatiotemporal delivery of nanoformulated liraglutide for cardiac regeneration after myocardial infarction, International journal of nanomedicine, 2017, 12:4835.
- Zhang, J., et al., Dual-targeting superparamagnetic iron oxide nanoprobes with high and low target density for brain glioma imaging, Journal of Colloid and Interface Science, 2016, V. 469, p. 86-92.
- Poojari, R., et al., Microtubule targeted therapeutics loaded polymeric assembled nanospheres for potentiation of antineoplastic activity, Faraday Discuss., 2016.
- Dasargyri, A., et al., Findings questioning the involvement of Sigma-1 receptor in the uptake of anisamide-decorated particles, Journal of Controlled Release, 2016, V. 224, p. 229-238.
- Alibolandi, M., et al., Folate receptor-targeted multimodal polymersomes for delivery of quantum dots and doxorubicin to breast adenocarcinoma: In vitro and in vivo evaluation, International Journal of Pharmaceutics, 2016, V. 500:1–2, p. 162-178.
- Bhargava-Shah, A., et al., Orlistat and antisense-miRNA-loaded PLGA-PEG nanoparticles for enhanced triple negative breast cancer therapy, Nanomedicine, 2016, 11: 3, P. 235-247.
- Spring, B.Q., et al., A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways, Nature nanotechnology, 2016.
- Akal, Z., et al., Superparamagnetic iron oxide conjugated with folic acid and carboxylated quercetin for chemotherapy applications, Ceramics International, 2016, 42(7):9065-72.
- Brinkman, A.M., et al., Aminoflavone-loaded EGFR-targeted unimolecular micelle nanoparticles exhibit anti-cancer effects in triple negative breast cancer. Biomaterials, 2016, 101:20-31.
- Li, Q., et al., Development of reactive oxygen species (ROS)-responsive nanoplatform for targeted oral cancer therapy. Journal of Materials Chemistry B, 2016.
- Cao, L.B., et al., Highly Stable PEGylated Poly (lactic-co-glycolic acid)(PLGA) Nanoparticles for the Effective Delivery of Docetaxel in Prostate Cancers, Nanoscale Research Letters, 2016, 11(1):1.
- Wang, C., et al., Multi-functionalized graphene oxide complex as a plasmid delivery system for targeting hepatocellular carcinoma therapy, RSC Advances, 2016, 6(27):22461-8.
- Thapa, R.K., et al., Liquid crystalline nanoparticles encapsulating cisplatin and docetaxel combination for targeted therapy of breast cancer, Biomaterials Science, 2016.
- Devulapally, R., et al., Polymer Nanoparticles Mediated Codelivery of AntimiR-10b and AntimiR-21 for Achieving Triple Negative Breast Cancer Therapy, ACS Nano, 2015, 9(3): 2290-2302.
- Liu, S., et al., Meter-long multiblock copolymer microfibers via interfacial bioorthogonal polymerization, Adv. Mater., 2015.
- Sun, N., et al., A Cellular Compatible Chitosan Nanoparticle Surface for Isolation and In Situ Culture of Rare Number CTCs, Small, 2015, 11: 5444–5451.
- Poojari, R., et al., A Chimeric Cetuximab-Functionalized Corona as a Potent Delivery System for Microtubule-Destabilizing Nanocomplexes to Hepatocellular Carcinoma Cells: A Focus on EGFR and Tubulin Intracellular Dynamics, Molecular Pharmaceutics, 2015, 12 (11), 3908-3923.
- Bielski, E. R., et al., Effect of the Conjugation Density of Triphenylphosphonium Cation on the Mitochondrial Targeting of Poly(amidoamine) Dendrimers, Molecular Pharmaceutics, 2015, 12 (8), 3043-3053.
- Devulapally, R., et al., Formulation of Anti-miR-21 and 4-Hydroxytamoxifen Co-loaded Biodegradable Polymer Nanoparticles and Their Antiproliferative Effect on Breast Cancer Cells, Molecular Pharmaceutics, 2015.
- Kang, T., et al., Enhancing Glioblastoma-Specific Penetration by Functionalization of Nanoparticles with an Iron-Mimic Peptide Targeting Transferrin/Transferrin Receptor Complex, Molecular Pharmaceutics, 2015, 12 (8), 2947-2961.
- Alibolandi, M., et al., Epithelial cell adhesion molecule aptamer conjugated PEG–PLGA nanopolymersomes for targeted delivery of doxorubicin to human breast adenocarcinoma cell line in vitro, International Journal of Pharmaceutics, 2015, 479:1, P. 241-251.
- Vandana, M., et al., Synergistic activity of combination therapy with PEGylated pemetrexed and gemcitabine for an effective cancer treatment, European Journal of Pharmaceutics and Biopharmaceutics, 2015, 94, P. 83-93.
- Lo, Y.L., et al., Folic Acid Linked Chondroitin Sulfate-Polyethyleneimine Copolymer Based Gene Delivery System, Journal of Biomedical Nanotechnology, 2015, 11:8, p. 1385-1400(16).
- Alibolandi, M., et al., In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer, Journal of Controlled Release, 2015, 209, P. 88-100.
- Terracciano, M., et al., Surface bioengineering of diatomite based nanovectors for efficient intracellular uptake and drug delivery, Nanoscale, 2015, 7, 20063-20074.
- Zhang, X.-Q., et al., Nanoparticles Containing a Liver X Receptor Agonist Inhibit Inflammation and Atherosclerosis. Advanced Healthcare Materials, 2015, 4: 228–236.
- Yang, L., et al., Photothermal Targetting therapy of A20 Mouse Lymphoma model using Anti CD-138 Antibody conjugated Gold Nanospheres, J Hematol Thrombo Dis, 2014, 2:3.
- Kang, T., et al., iNGR-modified PEG-PLGA nanoparticles that recognize tumor vasculature and penetrate gliomas. Biomaterials, 2014, 35(14): p. 4319-4332.
- Baker, D.W., et al., Development of optical probes for in vivo imaging of polarized macrophages during foreign body reactions. Acta Biomaterialia, 2014, 10(7): p. 2945-2955.
- Jain, S., et al., Combinatorial bio-conjugation of gemcitabine and curcumin enables dual drug delivery with synergistic anticancer efficacy and reduced toxicity, RSC Adv., 2014, 4, 29193-29201.
- Gu, W., et al., Chlorotoxin-conjugated, PEGylated Gd2O3 nanoparticles as a glioma-specific magnetic resonance imaging contrast agent, RSC Adv., 2014, 4, 50254-50260.
- Liu, Y.-S., Folate-mediated and doxorubicin-conjugated poly(ε-caprolactone)-g-chondroitin sulfate copolymers for enhanced intracellular drug delivery, RSC Adv., 2014, 4, 59548-59557.
- Zhou, W., et al., Aptamer-nanoparticle bioconjugates enhance intracellular delivery of vinorelbine to breast cancer cells, Journal of Drug Targeting, 2014, 22:1.
- Schuster, B.S.,et al., Overcoming the cystic fibrosis sputum barrier to leading adeno-associated virus gene therapy vectors, Mol Ther., 2014, 22(8):1484-93.
- Xiao, B., et al., Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-α RNA interference for IBD therapy, Biomaterials, 2013, 34(30), p:7471-7482.
- Sanna, V., et al, Resveratrol-Loaded Nanoparticles Based on Poly(epsilon-caprolactone) and Poly(d,l-lactic-co-glycolic acid)–Poly(ethylene glycol) Blend for Prostate Cancer Treatment, Mol. Pharmaceutics, 2013, 10(10), p:3871–3881.
- Han, H., et al., Targeted Nanoparticles Assembled via Complexation of Boronic-Acid-Containing Targeting Moieties to Diol-Containing Polymers, Bioconjugate Chem., 2013, 24(4), p: 669–677.
- Jain, R., et al., Antitumor activity of a monoclonal antibody targeting major histocompatibility complex class I–Her2 peptide complexes, Journal of the National Cancer Institute, 2013, 105.3, 202-218.
- Baker, D.W., The Pivotal Role Of Fibrocytes On Foreign Body Reactions, UTA, 2013.
- Vandana, M., et al., Reduced Folate Carrier Independent Internalization of PEGylated Pemetrexed: A Potential Nanomedicinal Approach for Breast Cancer Therapy, Mol. Pharmaceutics, 2012, 9(10), p: 2828–2843.
- Sanna, V., et al., Targeted Biocompatible Nanoparticles for the Delivery of (−)-Epigallocatechin 3-Gallate to Prostate Cancer Cells, Journal of Medicinal Chemistry, 2011, 54 (5), 1321-1332.
- Guo, J., et al., Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery, Biomaterials, 2011, 32:31, P. 8010-8020.
- Sanna, V., et al, Development of Polymeric Microbubbles Targeted to Prostate-Specific Membrane Antigen as Prototype of Novel Ultrasound Contrast Agents, Mol. Pharmaceutics, 2011, 8 (3), pp 748–757.
- Peng Zou, et al., Superparamagnetic Iron Oxide Nanotheranostics for Targeted Cancer Cell Imaging and pH-Dependent Intracellular Drug Release, Mol. Pharmaceutics, 2010, 7(6) p: 1974–1984.
- Biswal, B.K., et al., Development of a targeted siRNA delivery system using FOL-PEG-PEI conjugate, Molecular Biology Reports, 2010, 37:6, p. 2919-2926.
- Lu, W., et al., Targeted Photothermal Ablation of Murine Melanomas with Melanocyte-Stimulating Hormone Analog–Conjugated Hollow Gold Nanospheres, Clinical Cancer Research, 2009, 15; 876.
- Aumsuwan, N., et al., Attachment of Ampicillin to Expanded Poly(tetrafluoroethylene): Surface Reactions Leading to Inhibition of Microbial Growth, Biomacromolecules, 2008, 9(7), p: 1712–1718.
Note: Starting July 2016, Amine PEG Acetic Acid is the new name of the product Amine PEG Carboxyl (MW 1000 (NH2-PEG1000-COOH), MW 2000 (NH2-PEG2000-COOH), MW 3500 (NH2-PEG3500-COOH), MW 5000 (NH2-PEG5000-COOH), MW 7500 (NH2-PEG7500-COOH), MW 10000 (NH2-PEG10K-COOH) and MW 20000 (NH2-PEG20K-COOH)). JenKem Technology has revised the name of the product to better reflect the chemical structure, as many other PEG derivatives with a COOH group are offered in the 2016 JenKem USA catalog.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.