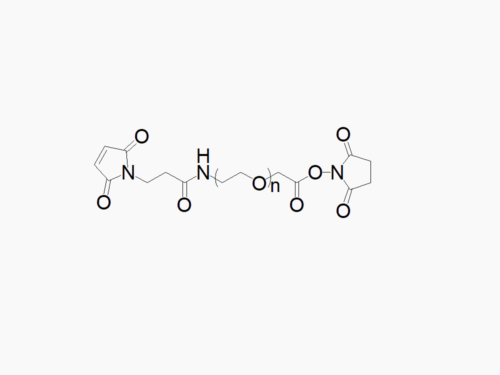
PEG products with additional MW may be made to order, please contact us for details
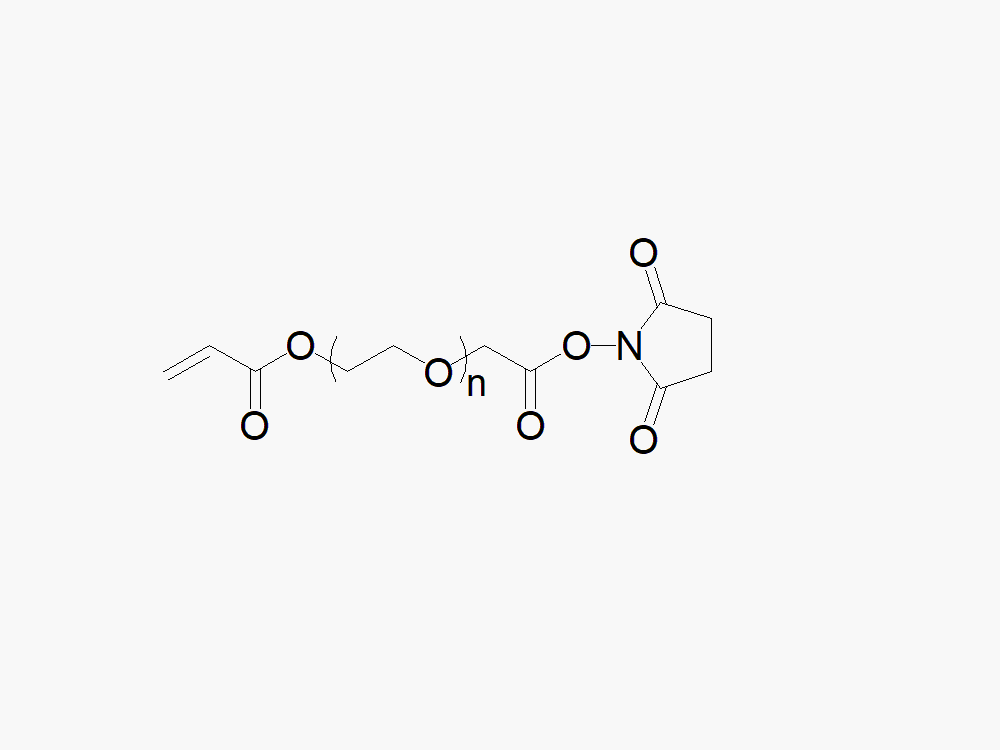
Acrylate PEG Succinimidyl Carboxymethyl Ester
$330.00 – $1,400.00
Description
High quality Acrylate PEG Succinimidyl Carboxymethyl Ester with a standard quality specification of >90% Substitution.
Heterobifunctional Acrylate PEG Succinimidyl Carboxymethyl Ester products from JenKem Technology are generally employed as crosslinking agents or as spacers between two different chemical entities. The PEG moiety in the heterofunctional PEG derivatives provides water solubility, biocompatibility, and flexibility. Applications are especially geared towards the development of antibody drug conjugates (ADC’s).
Heterobifunctional PEGylation reagents with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis. Please inquire at tech@jenkemusa.com about pricing and availability of custom PEGs.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Click here to download the MSDS
References:
- Colombani, T., et al., Hypoxia-inducing cryogels uncover key cancer-immune cell interactions in an oxygen-deficient tumor microenvironment, Bioactive Materials, 29, 2023.
- Liu, Z., et al., Modulated integrin signaling receptors of stem cells via ultra-soft hydrogel for promoting angiogenesis, Composites Part B: Engineering, 2022, 234.
- Rezaeeyazdi, M., et al., Engineering hyaluronic acid-based cryogels for CD44-mediated breast tumor reconstruction, Materials Today Bio, 2022, p.100207.
- Ghuman, H., et al., ECM hydrogel improves the delivery of PEG microsphere-encapsulated neural stem cells and endothelial cells into tissue cavities caused by stroke, Brain Research Bulletin, 2021, V. 168, P. 120-137.
- Wang, Y., et al., Minimally invasive co-injection of modular micro-muscular and micro-vascular tissues improves in situ skeletal muscle regeneration. Biomaterials. 2021.
- Li, X, et al., Holographic Display-Based Control for High-Accuracy Photolithography of Cellular Micro-Scaffold with Heterogeneous Architecture. IEEE/ASME Transactions on Mechatronics. 2021
- Li, X, et al., Automated Fabrication of the High-Fidelity Cellular Micro-Scaffold Through Proportion-Corrective Control of the Photocuring Process. IEEE Robotics and Automation Letters. 2021, 6(2):849-54.
- Nam, K, et al., Anisotropically Functionalized Aptamer-DNA Nanostructures for Enhanced Cell Proliferation and Target-Specific Adhesion in 3D Cell Cultures. Biomacromolecules. 2021.
- Post, A., et al., Elucidation of Endothelial Cell Hemostatic Regulation with Integrin-Targeting Hydrogels, Annals of biomedical engineering, 2019.
- Shih, T.Y., et al., Injectable, Tough Alginate Cryogels as Cancer Vaccines, Advanced healthcare materials, 2018, p.1701469.
- Stukel, J.M., et al., The interplay of peptide affinity and scaffold stiffness on neuronal differentiation of neural stem cells. Biomedical Materials, 2018, 13(2), p.024102.
- Post, A., et al., Introduction of sacrificial bonds to hydrogels to increase defect tolerance during suturing of multilayer vascular grafts, Acta Biomaterialia 2018, V. 69, p. 313-322.
- Lauridsen, H.M., et al., Ultrathin and elastic hydrogels regulate human neutrophil extravasation across endothelial-pericyte bilayers, PloS one, 2017, 12(2).
- Slaughter, G., et al., Fabrication of palladium nanowire array electrode for biofuel cell application, Microelectronic Engineering, 2016, 149, P. 92-96.
- Wang, Y., et al., Electroresponsive Nanoparticles Improve Antiseizure Effect of Phenytoin in Generalized Tonic-Clonic Seizures. Neurotherapeutics, 2016, 1-1.
- Ho-Joon Lee, et al., Poly(ethylene glycol) diacrylate/hyaluronic acid semi-interpenetrating network compositions for 3-D cell spreading and migration, Acta Biomaterialia, 2015, 14, p: 43-52.
- Parmar, P. A., et al., Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration, Biomaterials, 2015, 54: 213-225.
- Hoffman, M.D., et al., Emulating native periosteum cell population and subsequent paracrine factor production to promote tissue engineered periosteum-mediated allograft healing, Biomaterials, 2015, 52, P. 426-440.
- Rich, M.H., et al., Water–Hydrogel Binding Affinity Modulates Freeze-Drying-Induced Micropore Architecture and Skeletal Myotube Formation, Biomacromolecules, 2015, 16 (8), 2255-2264.
- Bae, S., et al., Cell-Mediated Dexamethasone Release from Semi-IPNs Stimulates Osteogenic Differentiation of Encapsulated Mesenchymal Stem Cells, Biomacromolecules, 2015, 16 (9), 2757-2765.
- Shkumatov, A., et al., Matrix stiffness-modulated proliferation and secretory function of the airway smooth muscle cells, American Journal of Physiology – Lung Cellular and Molecular Physiology, 2015, 308:11, L1125-L1135.
- Mahadik, B.P., et al., The use of covalently immobilized stem cell factor to selectively affect hematopoietic stem cell activity within a gelatin hydrogel, Biomaterials, 2015, 67, P. 297-307.
- Atanu, S., et al., Capillary Channel Polymer Fiber-Based Scaffolds for Neural Regeneration, 2015.
- Reis, L.A., et al., A Peptide Modified Hydrogel Therapy for Acute Myocardial Infarction, 2015.
- Kim, H. D., et al., Extracellular-Matrix-Based and Arg-Gly-Asp–Modified Photopolymerizing Hydrogels for Cartilage Tissue Engineering, Tissue Engineering Part A, 2015, 21(3-4): 757-766.
- Bencherif, S.A., et al., Injectable cryogel-based whole-cell cancer vaccines, Nature Communications, 2015, 6: 7556.
- Dang, L.T.H., et al., Inhibition of apoptosis in human induced pluripotent stem cells during expansion in a defined culture using angiopoietin-1 derived peptide QHREDGS, Biomaterials, 2014, 35(27): p. 7786-7799.
- Hoffman, M.D., et al., Degradable hydrogels for spatiotemporal control of mesenchymal stem cells localized at decellularized bone allografts. Acta Biomaterialia, 2014, 10(8): p. 3431-3441.
- Gonzalez, A. L., et al., Integrin-driven monocyte to dendritic cell conversion in modified extracorporeal photochemotherapy, Clin Exp Immunol, 2014, 175(3): 449-457.
- Slaughter, G., et al., Fabrication of Enzymatic Glucose Hydrogel Biosensor Based on Hydrothermally Grown ZnO Nanoclusters, Sensors Journal, IEEE, 2014, 14:5, pp.1573-1576.
- Feric, N.T., et al., Angiopoietin-1 peptide QHREDGS promotes osteoblast differentiation, bone matrix deposition and mineralization on biomedical materials, Biomater. Sci., 2014, 2, 1384-1398.
- Shkumatov, A., et al., Matrix Rigidity-Modulated Cardiovascular Organoid Formation from Embryoid Bodies, PLoS ONE, 2014, 9(4): e94764.
- Luong, P.T., et al, Drying and storage effects on poly(ethylene glycol) hydrogel mechanical properties and bioactivity. J Biomed Mater Res Part A, 2014, 102A, 3066–3076.
- Kim, J., et al., Cell-Friendly Inverse Opal-Like Hydrogels for a Spatially Separated Co-Culture System. Macromol. Rapid Commun., 2014, 35: 1578–1586.
- Chan, V., 3D Printing of Biological Machines for Biology and Medicine, University of Illinois at Urbana-Champaign, 2013.
- Qi, H., et al., DNA-directed self-assembly of shape-controlled hydrogels. Nat Commun, 2013, 4.
- Browning, M.B., et al., Bioactive Hydrogels with Enhanced Initial and Sustained Cell Interactions, Biomacromolecules, 2013, 14(7) p: 2225–2233
- Hoffman, M. D., et al., Agonism of Wnt-beta-catenin signalling promotes mesenchymal stem cell (MSC) expansion, J Tissue Eng Regen Med, 2013.
- Hoffman, M. D., et al., The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing. Biomaterials, 2013, 34.35: 8887-8898.
- Reid, B., et al., Enhanced tissue production through redox control in stem cell-laden hydrogels, Tissue Engineering Part A, 2013, 19.17-18, 2014-2023.
- Zhou, W., et al., Comparison of neurite growth in three dimensional natural and synthetic hydrogels. Journal of Biomaterials Science, Polymer Edition, 2013, 24.3: 301-314.
- Scott, M.A., Ultra-rapid 2-D and 3-D laser microprinting of proteins. Diss. Massachusetts Institute of Technology, 2013.
- Browning, M.B., et al., Development of a Biostable Replacement for PEGDA Hydrogels, Biomacromolecules, 2012, 13(3), p: 779–786.
- Wang, X., et al., Endothelial cell adhesion and proliferation to PEGylated polymers with covalently linked RGD peptides, J Biomed Mater Res A, 2012, 100(3): 794-801.
- Browning, M.B., et al., Multilayer vascular grafts based on collagen-mimetic proteins, Acta Biomaterialia, 2012, 8(3), p: 1010-1021.
- Chen, A.A., at al., Humanized mice with ectopic artificial liver tissues, PNAS, 2011, 108(29), p: 11842–11847.
- Cao Y., et al., A peptidomimetic inhibitor of matrix metalloproteinases containing a tetherable linker group, J Biomed Mater Res A., 2011, 96(4):663-72.
- Xu, X., et al., Shape Memory RGD-Containing Networks: Synthesis, Characterization, and Application in Cell Culture. Macromol. Symp., 2011, 309-310: 162–172.
- Yang, L., et al., Design considerations in the use of interdigitated microsensor electrode arrays (IMEs) for impedimetric characterization of biomimetic hydrogels, Biomedical Microdevices, 2011, 13:2, pp 279-289.
- Zorlutuna, P., et al., Stereolithography-Based Hydrogel Microenvironments to Examine Cellular Interactions, Adv. Funct. Mater., 2011, 21: 3642–3651.
- Justin, G., et al., Electroconductive Blends of Poly(HEMA-co-PEGMA-co-HMMAco-SPMA) and Poly(Py- co-PyBA): In Vitro Biocompatibility, Journal of Bioactive and Compatible Polymers, 2010, 25: 121-140.
- Cosgriff-Hernandez, E., et al., Bioactive hydrogels based on Designer Collagens, Acta Biomaterialia, 2010, 6(10), p: 3969-3977.
- Yang, X., et al., Multifunctional Stable and pH-Responsive Polymer Vesicles Formed by Heterofunctional Triblock Copolymer for Targeted Anticancer Drug Delivery and Ultrasensitive MR Imaging, ACS Nano, 2010, 4(11) p: 6805–6817.
- Reis, L.G., et al., Modification of Hydrogel Scaffolds for the Modulation of Corneal Epithelial Cell Responses, 26th Southern Biomedical Engineering Conference SBEC, 2010, 32, pp 175-179.
- Chan, V., et al., Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation, Lab Chip, 2010, 10, 2062-2070.
- He, J., et al., Rapid Generation of Biologically Relevant Hydrogels Containing Long-Range Chemical Gradients, Adv. Funct. Mater., 2010, 20: 131–137.
- Justin, G., et al., Characterization of Electroconductive Blends of Poly(HEMA-co-PEGMA-co-HMMA-co-SPMA) and Poly(Py-co-PyBA), Biomacromolecules, 2009, 10 (9), 2539-2549.
- Boztas, A.O., et al., Immobilization and Release of the Redox Mediator Ferrocene Monocarboxylic Acid from within Cross-Linked p(HEMA-co-PEGMA-co-HMMA) Hydrogels, Biomacromolecules, 2009, 10(8) p: 2135–2143.
- Justin, G., et al., Bioactive Hydrogel Layers on Microdisk Electrode Arrays: Cyclic Voltammetry Experiments and Simulations. Electroanalysis, 2009, 21: 1125–1134.
- Rahman, A., et al., Bioactive Hydrogel Layers on Microdisk Electrode Arrays: Impedimetric Characterization and Equivalent Circuit Modeling. Electroanalysis, 2009, 21: 1135–1144.
- Justin, G., et al., Biomimetic hydrogels for biosensor implant biocompatibility: electrochemical characterization using micro-disc electrode arrays (MDEAs), Biomedical Microdevices, 2009, 11:1, pp 103-115.
Note: Starting July 2016, Acrylate PEG Succinimidyl Carboxymethyl Ester is the new name of the product Acrylate PEG NHS Ester (MW 2000 (ACLT-PEG2000-NHS), MW 3500 (ACLT-PEG3500-NHS) MW 5000 (ACLT-PEG5000-NHS) and MW 7500 (ACLT-PEG7500-NHS)). JenKem Technology has revised the name of the product to better reflect the chemical structure.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.