 JenKem Technology manufactures high quality activated PEGs for Amine PEGylation with NHS and Carboxyl functionalities, with high purity and low polydispersity.
JenKem Technology manufactures high quality activated PEGs for Amine PEGylation with NHS and Carboxyl functionalities, with high purity and low polydispersity.
Structurally, JenKem Technology offers amine reactive linear PEGs, Y-shape PEGs, U-shape PEGs, multiarm PEGs, and monodisperse PEGs. The sterically bulky structure of JenKem Technology’s proprietary Y-shape branched PEG derivatives, consisting of two linear methoxy PEG chains attached to a central core with an active NHS group, may help to reduce the number of attachment sites to a protein molecule.
PEG NHS Esters with stable linker: MPEG-SCM, MPEG-SPA, MPEG-SBA, MPEG-SHA, MPEG-SGA, MPEG-SSA, SCM-PEG-SCM, Y-PEG-NHS, MPEG2-LYS-PEG-NHS, GLUC-PEG-NHS, GALA-PEG-NHS
PEG products with carbonate linker: MPEG-SC, MPEG-NPC
Degradable PEGs with cleavable NHS ester linker: MPEG-SS, SS-PEG-SS, and MPEG-SG
PEG Carboxyl: Y-PEG-COOH, MPEG-CM, MPEG-PA, MPEG5-PA, MPEG6-PA, MPEG-BA, MPEG-HA – more stable than NHS PEGs.
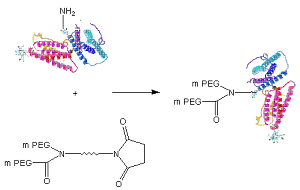
Schematic of Protein PEGylation with JenKem® Y-PEG-NHS
Amine PEGylation products with molecular weights, branching, and functional groups not listed in our online catalog may be available by custom synthesis, along with PEGylation services. JenKem Technology provides GMP grade PEG derivatives and bulk orders via custom synthesis, offering the opportunity to match customers’ special quality requirements.
JenKem Technology is capable of development and synthesis of a wide range of GMP PEG derivatives in 200g to 40 kg or greater batches, under ISO 9001 and ISO 13485 certified quality management system, following ICH Q7A guidelines. In 2020 JenKem Technology will transition to use High Purity (> 99%) mPEG Raw Materials to manufacture GMP grade Methoxy PEG Derivatives. For inquiries on cGMP production of PEG derivatives please contact us at tech@jenkemusa.com.
For global distribution, please visit link. Click the buttons below to order directly from JenKem Technology:
Y-shaped NHS PEGs with Stable Linker
| PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | Y-shape PEG NHS is reactive towards the amino group of lysine(s) on proteins or other biologics. Amine PEGylation with Y-shape PEG NHS can be completed in less than 1hr at pH 7-8. JenKem proprietary Y-shape PEGs are more selective, due to their sterically bulky structure and have been employed in PEGylation of nanoparticles, Cp40, DNA aptamer (SOMAmers), G-CSF, Gentamicin, IFN-α2a, IFN-α2b, LIF receptor antagonist (LA), L-RNA (Spiegelmers), rhGH, and TNF-α, among others. [4, 25-29] |
U-shaped Branched NHS PEGs with Stable Linker
| PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | MPEG2-NHS Ester is reactive towards the amino group of lysine(s), such as amines present on interferon alpha-2a, or aptamers. |
Linear Methoxy NHS PEGs with Stable Linker
| PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | Methoxy PEG Succinimidyl Carboxymethyl Ester reacts with the amine group of lysine(s) at room temperature in less than 1hr at pH 7-8. Shorter hydrolysis half life of M-PEG-SCM ensures maximum selectivity towards most sterically available amine groups. [6-8] | |
| ≥ 95% | Methoxy PEG Succinimidyl Propionate (M-SPA) reacts with the amino group of lysine(s) on proteins or other biologics, such as growth hormone B2036, granulocyte colony stimulating factor MAXY-G34, uricase, [33, 34]. M-SPA has a longer hydrolysis half-life compared with M-PEG-SCM [12]. | |
| ≥ 95% | Methoxy PEG Succinimidyl Butanoate reacts with the amine group of lysine(s) such as the lysines on EPO, at room temperature at pH 7-8. Methoxy PEG Succinimidyl Butanoate has a longer hydrolysis half-life compared with M-PEG-SCM. | |
| ≥ 95% | Methoxy PEG Succinimidyl Hexanoate reacts with the amine group of lysine(s) at pH 7-8. Methoxy PEG Succinimidyl Hexanoate has a longer hydrolysis half-life compared with M-PEG-SCM and M-PEG-SBA. [9, 31, 32] | |
| ≥ 95% | Methoxy PEG Succinimidyl Succinamide reacts with the amine group of lysine(s) at pH 7-8. Methoxy PEG Succinimidyl Succinamide has a longer hydrolysis half-life compared with M-PEG-SCM.[10] | |
| ≥ 90% | Methoxy PEG Succinimidyl Glutaramide reacts with the amine group of lysine(s) at pH 7-8. Methoxy PEG Succinimidyl Glutaramide has a longer hydrolysis half-life compared with M-PEG-SCM. [11] |
Linear NHS PEGs with Cleavable Linker
| PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | Methoxy PEG Succinimidyl Succinate is a degradable PEG linker reacting with the amino group of lysine(s) on proteins or other biologics, such as L-asparaginase, adenosine deaminase, at pH 7-8, while the ester linkage is cleaved under regular ester cleaving reaction conditions.[1,2] | |
| ≥ 95% | Methoxy PEG Succinimidyl Glutarate is a degradable PEG linker that reacts with the amino group of lysine(s) on proteins or other biologics at pH 7-8, while the ester linkage is cleaved under regular ester cleaving reaction conditions. |
Linear Carbonate PEGs
| PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | Methoxy PEG Succinimidyl Carbonate reacts with the amino group of lysine(s) on proteins or other biologics, such as the lysines present on IFN, Interferon alpha 2b. Methoxy PEG Succinimidyl Carbonate has a longer hydrolysis half-life compared with M-PEG-SCM.[3, 35]. | |
| ≥ 90% | Methoxy PEG Nitrophenyl Carbonate is reactive towards the the amino group of lysine(s) on proteins or other biologics, with a longer hydrolysis half-life compared with M-PEG-SCM. |
Linear Monosaccharide NHS PEGs
| PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 90% | Galactose PEG NHS reacts with amino group of lysine(s) at room temperature in under 1hr, at pH 7-8. The presence of the galactose monosaccharide increases significantly the selectivity of the PEGylation reaction. [5] | |
| ≥ 90% | Glucose PEG NHS reacts with amine group of lysine(s) at room temperature in less than 1hr, at pH 7-8. The presence of the monosaccharide sugar group (Glucose) increases significantly the selectivity of the PEGylation reaction. [30] |
Y-shaped Branched PEG Carboxyl
| PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | Y-shape PEG Carboxyl from JenKem Technology is reactive towards the amino group of lysine(s) at pH 7-8. |
Linear Carboxyl PEGs
| PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| ≥ 95% | Methoxy PEG Acetic Acid (M-COOH or M-CM) is an amine reactive reagent. Methoxy PEG Carboxyl is more stable than M-PEG-SCM.[15-17] | |
| ≥ 95% | Monodisperse Methoxy PEG Propionic Acid is more stable than M-PEG-SCM. JenKem Technology’s monodisperse discrete PEG Propionic Acid products are produced via very reproducible chemical reactions and lack the polydispersity of traditional PEG polymers. Rective towards the amino group of lysine(s) on proteins or other biologics. [18] | |
| ≥ 90% | Methoxy PEG Propionic Acid (M-PA, or MPEG Propanoic Acid) is more stable than M-PEG-SPA. [37] | |
| ≥ 90% | Methoxy PEG Butanoic Acid (M-BA) is more stable than M-PEG-SBA. | |
| ≥ 95% | Methoxy PEG Hexanoic Acid is more stable than M-PEG-SHA. |
Linear Homobifunctional PEGs for Amine PEGylation
| PEG PRODUCT | SUBSTITUTION | REACTIVITY DETAILS |
|---|---|---|
| > 90% | SS PEG SS PEG (Succinimidyl Succinate)2 Amino reactive PEG diSS ester crosslinker for the amino group of lysine(s) on proteins or other biologics, featuring cleavable ester. | |
| ≥ 95% | SCM PEG SCM (or NHS-PEG-NHS, PEG (Succinimidyl Carboxymethyl)2) Amino reactive PEG diNHS ester crosslinker for the amino group of lysine(s) on proteins or other biologics [23]. | |
| ≥ 95% | Carboxyl PEG Carboxyl (CM-PEG-CM, or Acetic Acid PEG Acetic Acid). Amino reactive PEG crosslinker. [19-22] | |
| ≥ 90% | Monodisperse Carboxyl PEG Carboxyl (Discrete CM-PEG-CM, discrete Acetic Acid PEG Acetic Acid, or discrete PEG di-acetic acid). Amino reactive PEG crosslinker. | |
| ≥ 90% | Monodisperse Propionic Acid PEG Propionic Acid (Discrete PA-PEG-PA, discrete PEG di-propionic acid). Amino reactive PEG crosslinker. |
Linear Heterobifunctional PEGs Functionalized with Carboxyl or NHS
Multiarm Homofunctional PEGs Functionalized with Carboxyl or NHS
Multiarm Heterobifunctional PEGs Functionalized with Carboxyl or NHS
References
- Xiong, Q., et al., Facile fabrication of reduction-responsive supramolecular nanoassemblies for co-delivery of doxorubicin and sorafenib towards hepatoma cells, Frontiers in pharmacology, 2018, 9, p.61.
- Mastorakos, P., et al., Biodegradable brain-penetrating DNA nanocomplexes and their use to treat malignant brain tumors, Journal of Controlled Release, 2017, 262, P. 37-46.
- Vaillard, V.A., et al., mPEG–NHS carbonates: Effect of alkyl spacers on the reactivity: Kinetic and mechanistic insights, Journal of Applied Polymer Science, 2019, 136(5):47028.
- Guo, L., et al., Application Instructions for Y-NHS-40K for Amine PEGylation, link.
- Luan, J., et al., GSDMD membrane pore is critical for IL-1β release and antagonizing IL-1β by hepatocyte-specific nanobiologics is a promising therapeutics for murine alcoholic steatohepatitis, Biomaterials, 2020, V. 227.
- Wang, P., et al., Precise gene delivery systems with detachable albumin shell remodeling dysfunctional microglia by TREM2 for treatment of Alzheimer’s disease, Biomaterials, 2022, V. 281.
- Chen, L., Ultra-small MoS2 nanodots-incorporated mesoporous silica nanospheres for pH-sensitive drug delivery and CT imaging, Journal of Materials Science & Technology, 2021, V. 63, P. 91-96.
- Yan, J., et al., Tumor Contrast Imaging with Gas Vesicles by Circumventing the Reticuloendothelial System, Ultrasound in Medicine & Biology, 2020, V. 46(2); P. 359-368.
- Mesken, J., et al., Modifying plasmid-loaded HSA-nanoparticles with cell penetrating peptides–Cellular uptake and enhanced gene delivery, International journal of pharmaceutics, 2017, 522(1):198-209.
- Li, C., et al., Real-Time Monitoring Surface Chemistry-Dependent In Vivo Behaviors of Protein Nanocages via Encapsulating an NIR-II Ag2S Quantum Dot, ACS Nano, 2015, 9 (12), 12255-12263.
- Suarez, et l., Intramyocardial injection of hydrogel with high interstitial spread does not impact action potential propagation, Acta Biomaterialia, 2015, V. 26, P. 13-22.
- Pfister, D., et al., Integrated process for high conversion and high yield protein PEGylation, Biotechnol. Bioeng., 2016.
- Goffin V, et al., Pegvisomant Pfizer/Sensus. Curr Opin Investig Drugs, 2004, 5:463–468.
- https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI%20Thesaurus&code=C71718
- Liu, Y., et al., Cathodic protected Mn2+ by NaxWO3 nanorods for stable magnetic resonance imaging-guided tumor photothermal therapy, Biomaterials, 2020, V. 234.
- Javanmardi, S., et al., Redox-Sensitive, PEG-Shielded Carboxymethyl PEI Nanogels Silencing MicroRNA-21, Sensitizes Resistant Ovarian Cancer Cells to Cisplatin, Asian Journal of Pharmaceutical Sciences, 2018.
- Jones, S.K., et al., Revisiting the value of competition assays in folate receptor-mediated drug delivery, Biomaterials, 2017.
- Zhang, C., Impact of Large Aggregated Uricases and PEG Diol on Accelerated Blood Clearance of PEGylated Canine Uricase, PLoS One, 2012, 7(6): e39659.
- Wang, C., et al., Synthesis and formation mechanism of bone mineral, whitlockite nanocrystals in tri-solvent system, Journal of Colloid and Interface Science, 2020, V. 569, P. 1-11.
- Ji, F., et al., A Dual pH/Magnetic Responsive Nanocarrier Based on PEGylated Fe3O4 Nanoparticles for Doxorubicin Delivery, Journal of Nanoscience and Nanotechnology, 2018, 18.7: 4464-4470.
- Xiao, S., et al., Aptamer-mediated gene therapy enhanced antitumor activity against human hepatocellular carcinoma in vitro and in vivo, Journal of Controlled Release, 2017.
- Jing, P., et al, Enhanced growth inhibition of prostate cancer in vitro and in vivo by a recombinant adenovirus-mediated dual-aptamer modified drug delivery system, Cancer Letters, 2016, V. 383(2), P. 230-242.
- Lu, C., et al., Poly(ethylene glycol) crosslinked multi-armed poly(ε-benzyloxycarbonyl-L-lysine)s as super-amphiphiles: Synthesis, self-assembly, and evaluation as efficient delivery systems for poorly water-soluble drugs, Colloids and Surfaces B: Biointerfaces, 2019, 182.
- Duncan, R., et al., Polymer therapeutics—polymers as drugs, drug and protein conjugates and gene delivery systems: past, present and future opportunities, Journal of drug targeting, 2006, 14(6), 337-341.
- Masao, H., et al., Chemically Modified Interleukin-6 Aptamer Inhibits Development of Collagen-Induced Arthritis in Cynomolgus Monkeys, Nucleic Acid Therapeutics, 2015.
- Winship, A.L., Interleukin-11 alters placentation and causes preeclampsia features in mice, Proc Natl Acad Sci U S A., 2015, 112(52):15928-33.
- AlQahtani, A.D., et al., Production of “biobetter” glucarpidase variants to improve drug detoxification and antibody directed enzyme prodrug therapy for cancer treatment, European Journal of Pharmaceutical Sciences, 2019, V. 127, P. 79-91.
- Roccaro, Aldo M. et al., SDF-1 Inhibition Targets the Bone Marrow Niche for Cancer Therapy, Cell Reports , 9 (1), 2014, p: 118 – 128.
- Haruta, K., et al., A Novel PEGylation Method for Improving the Pharmacokinetic Properties of Anti-Interleukin-17A RNA Aptamers, Nucleic acid therapeutics, 2017, 27(1):36-44.
- Drappier, C., pH-Responsive Surface Shielding By Electrostatic Adsorption of PEG Segments With Anionic Chain End, Universite Bordeaux, 2013.
- Look, L., et al., Ligand-Modified Human Serum Albumin Nanoparticles for Enhanced Gene Delivery, Molecular Pharmaceutics, 2015, 12 (9), 3202-3213.
- Fahrlander E., et al., PEGylated human serum albumin (HSA) nanoparticles: preparation, characterization and quantification of the PEGylation extent, Nanotechnology, 2015, 26(14):145103.
- Hershfield, M.S., Set al. Development of PEGylated mammalian urate oxidase as a therapy for patients with refractory gout, PEGylated Protein Drugs: Basic Science and Clinical Applications. Birkhauser Basel, 2009, 217-227.
- Ma, G., PEGylated drugs: Concept, Design and Application, Science Press, 2016, 314.
- Ma, S.S., et al., The pharmacokinetic and pharmacodynamic properties of site-specific pegylated genetically modified recombinant human interleukin-11 in normal and thrombocytopenic monkeys, European Journal of Pharmaceutics and Biopharmaceutics, 2017.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021756s006,s007lbl.pdf
- Oddone, N., et al., ROS-responsive “smart” polymeric conjugate: Synthesis, characterization and proof-of-concept study, International Journal of Pharmaceutics, 2019, 570.
JenKem Technology specializes in the development and manufacture of high-quality polyethylene glycol (PEG) derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified and adheres to ICH Q7 guidelines for GMP manufacture. The production of JenKem® PEGs is back integrated to in-house polymerization from ethylene oxide to ensure batch to batch consistency. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.
